��Ŀ����
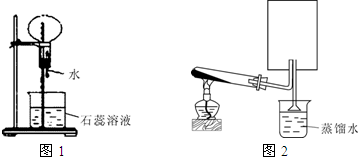
 A��һ�ְ�ɫ���壬����Ũ����������Һ���ȣ��ų���ɫ����B����Բ����ƿ�ռ������B������ͼװ����������ѹ�ιܵĽ�ͷʱ�����Եõ���ɫ��Ȫ��A��Ũ���ᷴӦ���ų���ɫ����C��C�ڿ��������γɰ�������Բ����ƿ�ռ������C������ͼװ����������ѹ�ιܵĽ�ͷʱ�����Եõ���ɫ��Ȫ��
A��һ�ְ�ɫ���壬����Ũ����������Һ���ȣ��ų���ɫ����B����Բ����ƿ�ռ������B������ͼװ����������ѹ�ιܵĽ�ͷʱ�����Եõ���ɫ��Ȫ��A��Ũ���ᷴӦ���ų���ɫ����C��C�ڿ��������γɰ�������Բ����ƿ�ռ������C������ͼװ����������ѹ�ιܵĽ�ͷʱ�����Եõ���ɫ��Ȫ����1��A�Ļ�ѧʽ��
NH4Cl
NH4Cl
��2�������ڳ�ȥB��ˮ�ֵĸ������
��ʯ��
��ʯ��
�ռ�����B�ķ����������ſշ�
�����ſշ�
��3��A��Ũ����������Һ��������B�Ļ�ѧ����ʽ
NH4Cl+NaOH
NH3��+H2O+NaCl
| ||
NH4Cl+NaOH
NH3��+H2O+NaCl
��
| ||
��������1�������⣺��ɫ����B��ʯ����Һ���γ���ɫ��Ȫ�����Ʋ�BΪ������AΪ��Σ���A��Ũ���ᷴӦ���ų���ɫ����C��C�ڿ��������γɰ�������CΪ�Ȼ��⣬����AΪ�Ȼ�泥�
��2�����ﰱ�����ü��Ը����������ݰ�����ˮ���ԡ��ܶ��ж��ռ�������
��3�����������Ӧ�õ�������ˮ��������д����ʽ��
��2�����ﰱ�����ü��Ը����������ݰ�����ˮ���ԡ��ܶ��ж��ռ�������
��3�����������Ӧ�õ�������ˮ��������д����ʽ��
����⣺��1����ɫ����B��ʯ����Һ���γ���ɫ��Ȫ�����Ʋ�BΪ������AΪ��Σ���A��Ũ���ᷴӦ���ų���ɫ����C��C�ڿ��������γɰ�������CΪ�Ȼ��⣬����AΪ�Ȼ�泥�
�ʴ�Ϊ��NH4Cl��
��2�����ﰱ�������ü�ʯ�������������������ˮ�����ܶ�С�ڿ�������������������ˮ����ֻ���������ſշ���
�ʴ�Ϊ����ʯ�ң������ſշ���
��3�������Ӧ�õ�������ˮ���Σ������Ȼ����Ũ����������Һ�������ɰ����Ļ�ѧ����ʽΪ��NH4Cl+NaOH
NH3��+H2O+NaCl��
�ʴ�Ϊ��NH4Cl+NaOH
NH3��+H2O+NaCl��
�ʴ�Ϊ��NH4Cl��
��2�����ﰱ�������ü�ʯ�������������������ˮ�����ܶ�С�ڿ�������������������ˮ����ֻ���������ſշ���
�ʴ�Ϊ����ʯ�ң������ſշ���
��3�������Ӧ�õ�������ˮ���Σ������Ȼ����Ũ����������Һ�������ɰ����Ļ�ѧ����ʽΪ��NH4Cl+NaOH
| ||
�ʴ�Ϊ��NH4Cl+NaOH
| ||
������������Ҫ���������ʵĻ�ѧ���ʣ��ѶȲ�������ѧ֪ʶ������ɣ�

��ϰ��ϵ�д�
 �����Ļ�������ҵϵ�д�
�����Ļ�������ҵϵ�д�
�����Ŀ
 �����仯������
�����仯������ ��ũҵ�����ȷ����й㷺Ӧ�ã��о�����������Ҫ���壮
��ũҵ�����ȷ����й㷺Ӧ�ã��о�����������Ҫ���壮
