��Ŀ����
����Ŀ�������������Ԫ�����ڱ���һ���֣���������Сд��ĸ�ֱ����һ�ֻ�ѧԪ�ء�
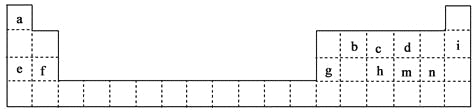
��1�������ϱ��ش��������⣺ ������Ԫ�����γ���������������_________����Ԫ�ط��ţ���m�������ӽṹʾ��ͼΪ_________��
��h��m��n���γɵ�����������ˮ�����������ǿ������˳��Ϊ_________���ѧʽ����
��d��e��f��g�γɵļ����ӵİ뾶��С�����˳��Ϊ_________�������ӷ�����д����
��2�������ܹ������Ƚ�m��n����Ԫ�طǽ�����ǿ������_________��
A���Ƚ�������Ԫ�ص���̬�⻯����ȶ���
B����������Ԫ�صĵ��ʷֱ�������������Һ��Ӧ
C����n�ĵ���ͨ�뵽m���⻯���ˮ��Һ��
��3���ֱ�д�����з�Ӧ�����ӷ���ʽ:
g��d�γɵĻ����� ��e��d��a�γɻ������ˮ��Һ��Ӧ
c����̬�⻯����������������Ӧ��ˮ���ﷴӦ
���𰸡���1����C��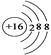 ����HClO4��H2SO4 ��H3 PO4����Al3+< Mg2+<Na+<O2-����2��AC����3��Al2O3+2OH��+3H2O��2 Al[(OH)4]����NH3+ H+��NH4+��
����HClO4��H2SO4 ��H3 PO4����Al3+< Mg2+<Na+<O2-����2��AC����3��Al2O3+2OH��+3H2O��2 Al[(OH)4]����NH3+ H+��NH4+��
�������������������1��������Ԫ����������C��ӦΪ���л����б��е�Ԫ�أ�mΪS���õ�2�����Ӵﵽ�ȶ��ṹ���������ӽṹʾ��ͼ��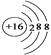 ���ǽ�����Խǿ��������������Ӧ��ˮ���������Խǿ��ͬ���ڴ������ҷǽ�������ǿ(ϡ���������)�����HClO4��H2SO4��H3PO4�������Ӳ�����ͬ���뾶����ԭ�������ĵ��������ͣ�˳����Al3��<Mg2��<Na��<O2������2���ǽ�����Խǿ�����⻯��Խ�ȶ�������ȷ��B���������绯��Ӧ������˵���ǽ�����ǿ�����ʴ���C�������û���Ӧ��������ǿ�İ������������û���������˵���ǽ����Ե�ǿ��������ȷ����3��g��d�γɵĻ�����ΪAl2O3��e��d��a�γɵĻ�����ΪNaOH��Al2O3������������������ӷ�Ӧ����ʽΪAl2O3��2OH��=2AlO2����H2O����Al2O3+2OH��+3H2O��2 Al[(OH)4]����c����̬�⻯��ΪNH3������������Ӧˮ������HNO3�����߷���NH3��H��=NH4����
���ǽ�����Խǿ��������������Ӧ��ˮ���������Խǿ��ͬ���ڴ������ҷǽ�������ǿ(ϡ���������)�����HClO4��H2SO4��H3PO4�������Ӳ�����ͬ���뾶����ԭ�������ĵ��������ͣ�˳����Al3��<Mg2��<Na��<O2������2���ǽ�����Խǿ�����⻯��Խ�ȶ�������ȷ��B���������绯��Ӧ������˵���ǽ�����ǿ�����ʴ���C�������û���Ӧ��������ǿ�İ������������û���������˵���ǽ����Ե�ǿ��������ȷ����3��g��d�γɵĻ�����ΪAl2O3��e��d��a�γɵĻ�����ΪNaOH��Al2O3������������������ӷ�Ӧ����ʽΪAl2O3��2OH��=2AlO2����H2O����Al2O3+2OH��+3H2O��2 Al[(OH)4]����c����̬�⻯��ΪNH3������������Ӧˮ������HNO3�����߷���NH3��H��=NH4����

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�