��Ŀ����
���ڹ�����ռ����Ҫ�ĵ�λ�����������̽����
��1��������������ˮ����ͨ�����ȵ�̿������ˮú����
C��s��+H2O��g��?H2��g��+CO��g����H=+131.3kJ����S=+133.7J/K
�÷�Ӧ�ڵ������ܷ��Է� ����ܻ��
��2����֪��400��ʱ��N2 ��g��+3H2��g��?2NH3��g����K=0.5��
��2NH3��g��?N2 ��g��+3H2��g����K= ������ֵ����
��400��ʱ����0.5L�ķ�Ӧ�����н��кϳɰ���Ӧ��һ��ʱ����N2��H2��NH3�����ʵ����ֱ�Ϊ2mol��1mol��2mol�����ʱ��ӦV��N2���� V��N2���������������=������ȷ����
��3����������ͬ�����и�����1molN2��3molH2����ijһ��ͬ�����·�Ӧ���ﵽƽ�⣬�������������ʱ��仯������ͼ������˵����ȷ���� ������ţ���
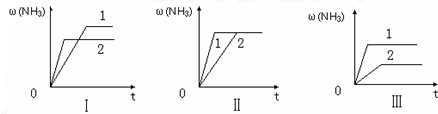
A��ͼ������Dz�ͬѹǿ�Է�Ӧ��Ӱ�죬��P2��P1
B��ͼ������Dz�ͬѹǿ�Է�Ӧ��Ӱ�죬��P1��P2
C��ͼ������Dz�ͬ�¶ȶԷ�Ӧ��Ӱ�죬��T1��T2
D��ͼ�������ͬ��ͬѹ�£��������ܣ�1��2��
��1��������������ˮ����ͨ�����ȵ�̿������ˮú����
C��s��+H2O��g��?H2��g��+CO��g����H=+131.3kJ����S=+133.7J/K
�÷�Ӧ�ڵ������ܷ��Է�
��2����֪��400��ʱ��N2 ��g��+3H2��g��?2NH3��g����K=0.5��
��2NH3��g��?N2 ��g��+3H2��g����K=
��400��ʱ����0.5L�ķ�Ӧ�����н��кϳɰ���Ӧ��һ��ʱ����N2��H2��NH3�����ʵ����ֱ�Ϊ2mol��1mol��2mol�����ʱ��ӦV��N2����
��3����������ͬ�����и�����1molN2��3molH2����ijһ��ͬ�����·�Ӧ���ﵽƽ�⣬�������������ʱ��仯������ͼ������˵����ȷ����

A��ͼ������Dz�ͬѹǿ�Է�Ӧ��Ӱ�죬��P2��P1
B��ͼ������Dz�ͬѹǿ�Է�Ӧ��Ӱ�죬��P1��P2
C��ͼ������Dz�ͬ�¶ȶԷ�Ӧ��Ӱ�죬��T1��T2
D��ͼ�������ͬ��ͬѹ�£��������ܣ�1��2��
��������1�����ݡ�G=��H-T��S�жϣ���G��0�Է����У���G��0���Է���
��2����ͬһ���淴Ӧ�������淴Ӧ��ƽ�ⳣ����Ϊ������
�ڼ���Ũ����Qc����ƽ�ⳣ���Ƚ��жϷ�Ӧ���з������ж����������ʹ�ϵ��
��3��A������ѹǿƽ�����ƣ�
B��ѹǿ��ͬ��ƽ��״̬��ͬ��
C�������¶�ƽ�������ƶ���
D��������Ӱ��ƽ���ƶ���
��2����ͬһ���淴Ӧ�������淴Ӧ��ƽ�ⳣ����Ϊ������
�ڼ���Ũ����Qc����ƽ�ⳣ���Ƚ��жϷ�Ӧ���з������ж����������ʹ�ϵ��
��3��A������ѹǿƽ�����ƣ�
B��ѹǿ��ͬ��ƽ��״̬��ͬ��
C�������¶�ƽ�������ƶ���
D��������Ӱ��ƽ���ƶ���
����⣺��1��C��s��+H2O��g��?H2��g��+CO��g����H=+131.3kJ����S=+133.7J/K����G=��H-T��S����G��0�Է����У���Ӧ���ʱ䡢�ر䶼�������¿��ܡ�G��0����Ӧ�Է����У��ʴ�Ϊ����
��2���ٷ�Ӧ2NH3��g��?N2 ��g��+3H2��g���ͷ�ӦN2 ��g��+3H2��g��?2NH3��g���ǻ�Ϊ���淴Ӧ����2NH3��g��?N2 ��g��+3H2��g����Kƽ�ⳣ��=
=2��
�ʴ�Ϊ��2��
��һ��ʱ���N2��H2��NH3�����ʵ����ֱ�Ϊ4mol/L��2mol/L��4mol/Lʱ��Qc=
=0.5�����Ը�״̬��ƽ��״̬�����淴Ӧ������ȣ�
�ʴ�Ϊ��=��
��3��A������ѹǿƽ�����ƣ������ĺ���Ӧ����ͼ����ʵ�ʲ�������A����
B��ѹǿ��ͬ��ƽ��״̬��ͬ����������ͬһƽ��״̬��ͼ����ʵ�ʲ�������B����
C�������¶�ƽ�������ƶ��������ĺ�����С��ͼ����ʵ�ʲ�������C����
D��������Ӱ��ƽ���ƶ�����ͼ���֪1����ƽ��ʱ����̣��ʴ�������1��2����D��ȷ��
�ʴ�Ϊ��D��
��2���ٷ�Ӧ2NH3��g��?N2 ��g��+3H2��g���ͷ�ӦN2 ��g��+3H2��g��?2NH3��g���ǻ�Ϊ���淴Ӧ����2NH3��g��?N2 ��g��+3H2��g����Kƽ�ⳣ��=
| 1 |
| 0.5 |
�ʴ�Ϊ��2��
��һ��ʱ���N2��H2��NH3�����ʵ����ֱ�Ϊ4mol/L��2mol/L��4mol/Lʱ��Qc=
| 42 |
| 4��23 |
| 42 |
| 4��22 |
�ʴ�Ϊ��=��
��3��A������ѹǿƽ�����ƣ������ĺ���Ӧ����ͼ����ʵ�ʲ�������A����
B��ѹǿ��ͬ��ƽ��״̬��ͬ����������ͬһƽ��״̬��ͼ����ʵ�ʲ�������B����
C�������¶�ƽ�������ƶ��������ĺ�����С��ͼ����ʵ�ʲ�������C����
D��������Ӱ��ƽ���ƶ�����ͼ���֪1����ƽ��ʱ����̣��ʴ�������1��2����D��ȷ��
�ʴ�Ϊ��D��
���������⿼�黯ѧƽ��ͼ����Ӱ�����ء���ѧƽ�ⳣ���ȣ��Ѷ��еȣ���2����ע�����ƽ�ⳣ������ʽ�������⣮

��ϰ��ϵ�д�
�����Ŀ
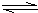 2NH3�ġ�H
2NH3�ġ�H