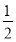��Ŀ����
��ԴΣ���ǵ�ǰȫ�����⣬��Դ������Ӧ����ԴΣ������Ҫ�ٴ롣
(1)����������������Դ����Դ����������________(�����)��
a��������չũ���������������Ľո�ת��Ϊ����Ч����Դ
b����������ú��ʯ�ͺ���Ȼ������������������������Դ����
c������̫���ܡ�ˮ�ܡ����ܡ������ܵ�����Դ������ʹ��ú��ʯ�͵Ȼ�ʯȼ��
d��������Դ���ģ�������Դ���ظ�ʹ�á���Դ��ѭ������
(2)���ʯ��ʯī��Ϊ̼��ͬ�������壬����ȼ����������ʱ����һ����̼�����ȼ�����ɶ�����̼����Ӧ�зų���������ͼ��ʾ��
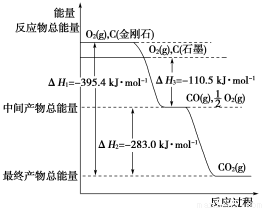
����ͨ��״���£����ʯ��ʯī��________(�������ʯ������ʯī��)���ȶ���ʯī��ȼ����Ϊ________��
��12 gʯī��һ����������ȼ�գ���������36 g���ù��̷ų�������________��
(3)��֪��N2��O2�����л�ѧ���ļ��ֱܷ���946 kJ��mol��1��497 kJ��mol��1��
N2(g)��O2(g)=2NO(g)����H��180.0 kJ��mol��1��
NO�����л�ѧ���ļ���Ϊ________kJ��mol��1��
(4)�ۺ������й���Ϣ����д��CO��NO��Ӧ���Ȼ�ѧ����ʽ_________________________________��
��(1)acd��(2)��ʯī��393.5 kJ��mol��1
��252.0 kJ��(3)631.5��(4)2NO(g)��2CO(g)=N2(g)��2CO2(g)����H����746.0 kJ��mol��1
����������(2)��ʯī���������ͣ����ȶ���ʯī��ȼ����ָ1 molʯī��ȫȼ������CO2ʱ�ų���������
��12 gʯī��24 g������Ӧ����1 mol C��0.75 mol O2��Ӧ��������0.5 mol CO��0.5 mol CO2���ų�����0.5 mol��110.5 kJ��mol��1��0.5 mol��393.5 kJ��mol��1��252.0 kJ��(3)��H��E(N2����)��E(O2����)��2E(NO����)��2E(NO����)��946 kJ��mol��1��497 kJ��mol��1��180.0 kJ��mol��1��E(NO����)��631.5 kJ��mol��1��(4)��֪��2CO(g)��O2(g)=2CO2(g)����H����566.0 kJ��mol��1��N2(g)��O2(g)=2NO(g)����H��180.0 kJ��mol��1��Ŀ�귴Ӧ2NO(g)��2CO (g)=N2(g)��2CO2(g)����ǰ������ã�����H����746.0 kJ��mol��1��

 ��ʦָ����ĩ��̾�ϵ�д�
��ʦָ����ĩ��̾�ϵ�д�