��Ŀ����
��A��B�����������ǵ������ͬ����Լ��85.7%��̼����A��Ħ������Ϊ56g/mol����Bʽ���ȿ�����ƽ��ʽ����С�������ʽ��A��ͬ����A��B����ʹ������Ȼ�̼��Һ��ɫ����������ʵ����ʵ�ش����⣮���ƶ�A��B�����Ļ�ѧʽ��
A______��B______
��A��B��______����ͬ���칹�壬д����ͬ�������ͬ���칹��Ľṹ��ʽ______
��д��B��������Ȼ�̼��Һ��Ӧ�Ļ�ѧ����ʽ��______
��B���Ծۺϳ�һ�ָ߷��ӻ����д���仯ѧ����ʽ��______
���𰸡��������ٸ�����A������̼���������������������Cԭ�ӡ�Hԭ����Ŀ��д��A�ķ���ʽ������A��B�����ʽ��ͬ���ٽ����Bʽ���ȿ�����ƽ��ʽ����С���ƶ�B�ķ���ʽ��
����A��B����ʹ������Ȼ�̼��Һ��ɫ�����в����ͼ�������A��B�ķ���ʽ��ṹ�ж��Ƿ����ͬ���칹�壬���ݽṹ������ʽ��д����������ͬ���칹�壻
����B��ʹ������Ȼ�̼��Һ��ɫ��˵��B�������ͼ�����Ϸ���ʽ�ж���ṹ��д������ʽ��
�ܸ�����B�Ľṹ��д��Ӧ����ʽ��
����⣺������̼Ԫ�ص���������Ϊ85.7%��������A��Cԭ�ӵ���ĿΪ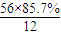 =4��������A��Hԭ�ӵ���ĿΪ
=4��������A��Hԭ�ӵ���ĿΪ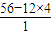 =8��������A�ķ���ʽΪC4H8��
=8��������A�ķ���ʽΪC4H8��
A��B�����ʽ��ͬ�����ʽΪCH2��Bʽ���ȿ�����ƽ��ʽ����С�����ʽCH2������B�ķ���ʽΪC2H4��
�ʴ�Ϊ��C4H8��C2H4��
��AΪC4H8��BΪC2H4�����߶���ʹ������Ȼ�̼��Һ��ɫ��˵�������к���1��C=C˫��������ϩ����
A����ͬ���칹�壬�У�CH2=CHCH2CH3��CH3CH=CHCH3��CH2=C��CH3��2
��B������ͬ���칹�壬
�ʴ�Ϊ��A��CH2=CHCH2CH3��CH3CH=CHCH3��CH2=C��CH3��2��
��B�ķ���ʽΪC2H4����ʹ������Ȼ�̼��Һ��ɫ����BΪ��ϩCH2=CH2������ϩ��Ӧ�ķ���ʽΪ��
CH2=CH2+Br2��CH2Br-CH2Br��
�ʴ�Ϊ��CH2=CH2+Br2��CH2Br-CH2Br��
����ϩ�����ۺϷ�Ӧ���ɾ���ϩ����Ӧ����ʽΪ��nCH2=CH2
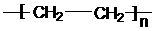 ��
��
�ʴ�Ϊ��nCH2=CH2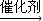
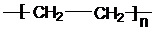 ��
��
���������⿼���л������ʽ�ƶϡ���������ͬ���칹����д��ϩ�������ʵȣ��ѶȲ���ע�����C���������������ƶϣ�
����A��B����ʹ������Ȼ�̼��Һ��ɫ�����в����ͼ�������A��B�ķ���ʽ��ṹ�ж��Ƿ����ͬ���칹�壬���ݽṹ������ʽ��д����������ͬ���칹�壻
����B��ʹ������Ȼ�̼��Һ��ɫ��˵��B�������ͼ�����Ϸ���ʽ�ж���ṹ��д������ʽ��
�ܸ�����B�Ľṹ��д��Ӧ����ʽ��
����⣺������̼Ԫ�ص���������Ϊ85.7%��������A��Cԭ�ӵ���ĿΪ
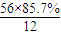 =4��������A��Hԭ�ӵ���ĿΪ
=4��������A��Hԭ�ӵ���ĿΪ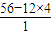 =8��������A�ķ���ʽΪC4H8��
=8��������A�ķ���ʽΪC4H8��A��B�����ʽ��ͬ�����ʽΪCH2��Bʽ���ȿ�����ƽ��ʽ����С�����ʽCH2������B�ķ���ʽΪC2H4��
�ʴ�Ϊ��C4H8��C2H4��
��AΪC4H8��BΪC2H4�����߶���ʹ������Ȼ�̼��Һ��ɫ��˵�������к���1��C=C˫��������ϩ����
A����ͬ���칹�壬�У�CH2=CHCH2CH3��CH3CH=CHCH3��CH2=C��CH3��2
��B������ͬ���칹�壬
�ʴ�Ϊ��A��CH2=CHCH2CH3��CH3CH=CHCH3��CH2=C��CH3��2��
��B�ķ���ʽΪC2H4����ʹ������Ȼ�̼��Һ��ɫ����BΪ��ϩCH2=CH2������ϩ��Ӧ�ķ���ʽΪ��
CH2=CH2+Br2��CH2Br-CH2Br��
�ʴ�Ϊ��CH2=CH2+Br2��CH2Br-CH2Br��
����ϩ�����ۺϷ�Ӧ���ɾ���ϩ����Ӧ����ʽΪ��nCH2=CH2
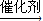
�ʴ�Ϊ��nCH2=CH2
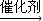
���������⿼���л������ʽ�ƶϡ���������ͬ���칹����д��ϩ�������ʵȣ��ѶȲ���ע�����C���������������ƶϣ�

��ϰ��ϵ�д�
�����Ŀ
