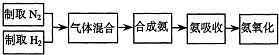
��Ŀ����
A��������
ijУ�Ƽ�С���ͬѧ�Dzɼ�������Ʒ��ÿ��һ��ʱ��ⶨ��Ʒ��pH���õ��������ݣ�
�ش��������⣺
��1���������ʱ��pH������õ���Ҫԭ��Ϊ
��2��ú�к�������̬������д��������һϵ�б仯�����������Ҫ�ɷֵ��йػ�ѧ����ʽ�������߲�һ��������
B����A��B�����������ǵ������ͬ����Լ��85.7%��̼����A������������ܶ���28����Bʽ���ȿ�����ƽ��ʽ����С�������ʽ��A��ͬ����A��B����ʹ������Ȼ�̼��Һ��ɫ����������ʵ����ʵ���㲢�ش����⣮
��1������A��B�����Ļ�ѧʽ�������㲽��д�����棩
A
��2��A��B��
��3��д��B��������Ȼ�̼��Һ��Ӧ�Ļ�ѧ����ʽ��
SO2
SO2
������������
����������
��ɢ�������У��ڿ���������������·���һϵ�б仯������ʱ����ˮ���գ�����ˮ��pHС��5.6
5.6
ʱ���γ������꣮ijУ�Ƽ�С���ͬѧ�Dzɼ�������Ʒ��ÿ��һ��ʱ��ⶨ��Ʒ��pH���õ��������ݣ�
| ʱ�� | ��ʼ | 8h�� | 16h�� | 24h�� | 32h�� | 40h�� | 48h�� |
| pH | 5.0 | 4.8 | 4.5 | 4.3 | 4.2 | 4.0 | 4.0 |
��1���������ʱ��pH������õ���Ҫԭ��Ϊ
��ˮ�������ᱻ���������ᣬ������ǿ
��ˮ�������ᱻ���������ᣬ������ǿ
����2��ú�к�������̬������д��������һϵ�б仯�����������Ҫ�ɷֵ��йػ�ѧ����ʽ�������߲�һ��������
S+O2
SO2
| ||
S+O2
SO2
��
| ||
2SO2+O2=2SO3
2SO2+O2=2SO3
��SO3+H2O�TH2SO4
SO3+H2O�TH2SO4
����
��
��B����A��B�����������ǵ������ͬ����Լ��85.7%��̼����A������������ܶ���28����Bʽ���ȿ�����ƽ��ʽ����С�������ʽ��A��ͬ����A��B����ʹ������Ȼ�̼��Һ��ɫ����������ʵ����ʵ���㲢�ش����⣮
��1������A��B�����Ļ�ѧʽ�������㲽��д�����棩
A
C4H8
C4H8
��BC2H4
C2H4
����2��A��B��
C4H8
C4H8
����A��B�ķ���ʽ������ͬ���칹�壮��3��д��B��������Ȼ�̼��Һ��Ӧ�Ļ�ѧ����ʽ��
CH2=CH2+Br2��CH2Br-CH2Br
CH2=CH2+Br2��CH2Br-CH2Br
��������A����������Ĺ涨��������
��1����ˮ�������ᱻ���������ᣮ
��2����ȼ�����ɶ�����������������Ϊ������������������ˮ�����������ᣮ
B����1��������A������������ܶ���28������A����Է����������ٸ���̼���������������������Cԭ�ӡ�Hԭ����Ŀ��д��A�ķ���ʽ��
����A��B�����ʽ��ͬ���ٽ����Bʽ���ȿ�����ƽ��ʽ����С���ƶ�B�ķ���ʽ��
��2������A��B�ķ���ʽ�жϣ�
��3����B��ʹ������Ȼ�̼��Һ��ɫ��˵��B�������ͼ�����Ϸ���ʽ�ж���ṹ��д������ʽ��
��1����ˮ�������ᱻ���������ᣮ
��2����ȼ�����ɶ�����������������Ϊ������������������ˮ�����������ᣮ
B����1��������A������������ܶ���28������A����Է����������ٸ���̼���������������������Cԭ�ӡ�Hԭ����Ŀ��д��A�ķ���ʽ��
����A��B�����ʽ��ͬ���ٽ����Bʽ���ȿ�����ƽ��ʽ����С���ƶ�B�ķ���ʽ��
��2������A��B�ķ���ʽ�жϣ�
��3����B��ʹ������Ȼ�̼��Һ��ɫ��˵��B�������ͼ�����Ϸ���ʽ�ж���ṹ��д������ʽ��
����⣺A��pH��5.6����ˮΪ���꣬SO2�͵����������ڴ����У��ڿ���������������·���һϵ�б仯������ʱ����ˮ�����γ����꣮
�ʴ�Ϊ��SO2�����������5.6��
��1����ˮ�������ᱻ���������ᣬ������ǿ��pHֵ��С��
�ʴ�Ϊ����ˮ�������ᱻ���������ᣬ������ǿ��
��2����ȼ�����ɶ�����������������Ϊ������������������ˮ�����������ᣮ��Ӧ����ʽ����Ϊ��
S+O2
SO2�� 2SO2+O2=2SO3�� SO3+H2O�TH2SO4��
�ʴ�Ϊ��S+O2
SO2�� 2SO2+O2=2SO3�� SO3+H2O�TH2SO4��
B����1����A������������ܶ���28��
������A����Է�������Ϊ28��2=56��
����̼Ԫ�ص���������Ϊ85.7%��
������A��Cԭ�ӵ���ĿΪ
=4
������A��Hԭ�ӵ���ĿΪ
=8
������A�ķ���ʽΪC4H8��
A��B�����ʽ��ͬ������A��B�����ʽΪCH2��
Bʽ���ȿ�����ƽ��ʽ����С��������ʽCH2������B�ķ���ʽΪC2H4��
��2��A�ķ���ʽΪC4H8������1-��ϩ��2-��ϩ���飬B�ķ���ʽΪC2H4��������ͬ���칹�壬����A����ͬ���칹�壮
�ʴ�Ϊ��C4H8��
��3��B�ķ���ʽΪC2H4����ʹ������Ȼ�̼��Һ��ɫ����BΪ��ϩCH2=CH2������ϩ��Ӧ�ķ���ʽΪ��
CH2=CH2+Br2��CH2Br-CH2Br��
�ʴ�Ϊ��CH2=CH2+Br2��CH2Br-CH2Br��
�ʴ�Ϊ��SO2�����������5.6��
��1����ˮ�������ᱻ���������ᣬ������ǿ��pHֵ��С��
�ʴ�Ϊ����ˮ�������ᱻ���������ᣬ������ǿ��
��2����ȼ�����ɶ�����������������Ϊ������������������ˮ�����������ᣮ��Ӧ����ʽ����Ϊ��
S+O2
| ||
�ʴ�Ϊ��S+O2
| ||
B����1����A������������ܶ���28��
������A����Է�������Ϊ28��2=56��
����̼Ԫ�ص���������Ϊ85.7%��
������A��Cԭ�ӵ���ĿΪ
| 56��85.7% |
| 12 |
������A��Hԭ�ӵ���ĿΪ
| 56-12��4 |
| 1 |
������A�ķ���ʽΪC4H8��
A��B�����ʽ��ͬ������A��B�����ʽΪCH2��
Bʽ���ȿ�����ƽ��ʽ����С��������ʽCH2������B�ķ���ʽΪC2H4��
��2��A�ķ���ʽΪC4H8������1-��ϩ��2-��ϩ���飬B�ķ���ʽΪC2H4��������ͬ���칹�壬����A����ͬ���칹�壮
�ʴ�Ϊ��C4H8��
��3��B�ķ���ʽΪC2H4����ʹ������Ȼ�̼��Һ��ɫ����BΪ��ϩCH2=CH2������ϩ��Ӧ�ķ���ʽΪ��
CH2=CH2+Br2��CH2Br-CH2Br��
�ʴ�Ϊ��CH2=CH2+Br2��CH2Br-CH2Br��
�������������ꡢ�л������ʽ�ƶϣ��ѶȲ���ע�������������ȷ��������ԭ����Ŀ��

��ϰ��ϵ�д�
�����Ŀ