��Ŀ����
����Ŀ��һ�������£������Ϊ3 L���ܱ������У�CO��H2��Ӧ���ɼ״�(CH3OH)(����ΪCu2O/ZnO)��CO(g)��2H2(g)![]() CH3OH(g)��������и��⣺
CH3OH(g)��������и��⣺
(1)��Ӧ�ﵽƽ��ʱ��ƽ�ⳣ������ʽ��K�� �������¶ȣ�Kֵ (����������������С������������)��
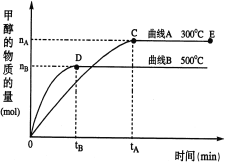
(2)��500�����ӷ�Ӧ��ʼ��ƽ�⣬v(H2)�� ��(����ͼ�г��ֵ���ĸ��ʾ)
(3)�������������������£��Դ���E�����ϵ���ѹ����ԭ����1/2�������йظ���ϵ��˵����ȷ���� (�����)��
a��H2��Ũ�ȼ���
b��CH3OH�����ʵ�������
c������Ӧ���ʼӿ죬�淴Ӧ����Ҳ�ӿ�
d������ƽ��ʱn(H2)/n(CH3OH)����
���𰸡�
(1)![]() ������
������
(2)![]() mol/(L��min)��(3)bc��
mol/(L��min)��(3)bc��
��������
���������(1)��ѧƽ�ⳣ��K����������Ũ����֮���뷴Ӧ��Ũ����֮���ıȣ�K=![]() ������ͼ֪�������¶ȼ״������ʵ�����С��ƽ�������ƶ���������Ӧ�Ƿ��ȷ�Ӧ���¶�Խ��ѧƽ�ⳣ��ԽС���ʴ�Ϊ��
������ͼ֪�������¶ȼ״������ʵ�����С��ƽ�������ƶ���������Ӧ�Ƿ��ȷ�Ӧ���¶�Խ��ѧƽ�ⳣ��ԽС���ʴ�Ϊ��![]() ����С��
������
(2)��500�����ӷ�Ӧ��ʼ��ƽ�⣬��n(CH3OH)=nBmol�����ݷ���ʽ֪���μӷ�Ӧ����n(H2)=2��n(CH3OH)=2nBmol��v(H2)=![]() =
= =
=![]() mol/(L��min)���ʴ�Ϊ��
mol/(L��min)���ʴ�Ϊ��![]() mol/(L��min)��
mol/(L��min)��
(3)a����С����൱������ѹǿ��ƽ�������ƶ�����������ת���ʣ���H2��Ũ������a����b������ѹǿƽ�������ƶ�����CH3OH�����ʵ������ӣ���b��ȷ��c����С����൱������ѹǿ�����淴Ӧ���ʶ�����c��ȷ��d������ѹǿƽ�������ƶ���������ƽ��ʱn(H2)/n(CH3OH)��С����d����ѡbc��


