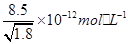��Ŀ����
��ͼ��ʾ�����������������ڲ�ͬpH�µ��ܽ�ȣ�S/mol��L��1��������˵������ȷ����


| A��pH��3ʱ��Һ����Ԫ�ص���Ҫ������ʽ��Fe3�� |
| B����Ni(NO3)2��Һ�к���������Co2�����ʣ���ͨ��������ҺpH�ķ�������ȥ |
| C����������Һ�е�Fe3����Cu2�����ɵ�����Һ��pH��4���� |
| D�����ں���Cu2����Ni2������Һ�м����ռNi(OH)2���ȳ��� |
C
�������������ͼ���֪��pH��3ʱ��Һ����Ԫ�ص���Ҫ������ʽ������������A����ȷ������ͼ���֪������������ȫ����ʱ��pH��������������ȫ����ʱ��pH�����Ե�Co2����ȫ����ʱNi2��Ҳ�Ѿ���ȫ������B����ȷ��pH��4ʱ����������ȫת��Ϊ����������������ͭ���ӻ�������Һ�У�C��ȷ��D����ȷ����Ϊͭ������ȫ����ʱ��pHС������������ȫ����ʱ��pH����ѡC��
�����������Ǹ߿��еij���ͼ���ѶȽϴ������ۺ���ǿ��������ͼ��������ѧ���̻���ֱ�����Ϣ�ǻ�ѧ���õı��﷽ʽ��ͼ������ӵ�нϴ����Ϣ�洢�����ܹ���ȫ��ؿ���ѧ���������Ƚϡ����������������������

��ϰ��ϵ�д�
�����Ŀ

 ��
�� �����й��ڲ�����֮��ת����˵���д�����ǣ�
�����й��ڲ�����֮��ת����˵���д�����ǣ�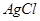 ������ˮ������ת��Ϊ
������ˮ������ת��Ϊ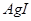
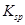 ���Խ�������Խ����ת��Ϊ�����ܵIJ�����
���Խ�������Խ����ת��Ϊ�����ܵIJ�����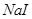 ��Һ�п�ʼת��Ϊ
��Һ�п�ʼת��Ϊ