��Ŀ����
��10�֣���ѭ���ֽ�ˮ������Ҫ�漰���з�Ӧ��
��. SO2��2H2O��I2===H2SO4��2HI
��. 2HI H2��I2
H2��I2
��. 2H2SO4===2SO2��O2��2H2O
(1) ����������Ӧ�������ж���ȷ���� ��
a����Ӧ�����ڳ����½���
b����Ӧ����SO2�����Ա�HIǿ
c��ѭ���������貹��H2O
d��ѭ�����̲���1 mol O2��ͬʱ����1 mol H2
(2) һ���¶��£���1 L�ܱ������м���1 mol HI(g)��������Ӧ��H2���ʵ�����ʱ��ı仯��ͼ��ʾ��
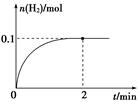
�� 0��2 min�ڵ�ƽ����Ӧ����v(HI)�� ��
�� ���¶��£�H2(g)��I2(g) 2HI(g)��ƽ�ⳣ��K�� ��
2HI(g)��ƽ�ⳣ��K�� ��
�� ��ͬ�¶��£�����ʼ����HI(g)�����ʵ�����ԭ����2������ ��ԭ����2����
a��ƽ�ⳣ�� b��HI��ƽ��Ũ��
c���ﵽƽ���ʱ�� d��ƽ��ʱH2���������
(3) ʵ������Zn��ϡ������ȡH2����Ӧʱ���������������Լ��е� ������H2�����ʽ�����
a��NaNO3 b��CuSO4 c��Na2SO4 d��NaHSO3
��. SO2��2H2O��I2===H2SO4��2HI
��. 2HI
 H2��I2
H2��I2��. 2H2SO4===2SO2��O2��2H2O
(1) ����������Ӧ�������ж���ȷ���� ��
a����Ӧ�����ڳ����½���
b����Ӧ����SO2�����Ա�HIǿ
c��ѭ���������貹��H2O
d��ѭ�����̲���1 mol O2��ͬʱ����1 mol H2
(2) һ���¶��£���1 L�ܱ������м���1 mol HI(g)��������Ӧ��H2���ʵ�����ʱ��ı仯��ͼ��ʾ��

�� 0��2 min�ڵ�ƽ����Ӧ����v(HI)�� ��
�� ���¶��£�H2(g)��I2(g)
 2HI(g)��ƽ�ⳣ��K�� ��
2HI(g)��ƽ�ⳣ��K�� ���� ��ͬ�¶��£�����ʼ����HI(g)�����ʵ�����ԭ����2������ ��ԭ����2����
a��ƽ�ⳣ�� b��HI��ƽ��Ũ��
c���ﵽƽ���ʱ�� d��ƽ��ʱH2���������
(3) ʵ������Zn��ϡ������ȡH2����Ӧʱ���������������Լ��е� ������H2�����ʽ�����
a��NaNO3 b��CuSO4 c��Na2SO4 d��NaHSO3
(1)c�� (2) ��0.1 mol��(L��min)��1�� ��64�� ��b (3)��b
�����������1��2SO2��O2��2H2O=2H2SO4�dz������Է����еģ�����2H2SO4===2SO2��O2��2H2O�����ڳ����½��У�a����SO2�ǻ�ԭ��HI�ǻ�ԭ�������SO2�Ļ�ԭ��ǿ��HI��b����������Ӧ���ܷ�ӦΪ2H2O
 2H2+O2������c��ȷ��d����
2H2+O2������c��ȷ��d������2����v(HI)=

�ڸ���ͼʾ��֪��ƽ��ʱc(H2)=0.1mol/L����c(I2)=0.1mol/L��c(HI)=0.8mol/L������K=
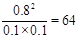 ��
��������ʼ����2molHI�����൱�ڽ�ԭ��������2����ѹǿ��ƽ�ⲻ�ƶ������������ʵ�Ũ����ԭ����2��������b��ȷ��
��3��Zn����CuSO4��Ӧ�γ�Cu��Znԭ��أ��ӿ췴Ӧ���ʣ�����b��ȷ��
�����������ۺ���ǿ�������ѶȲ�����һ��Ľ�����ɺ����ɽ��

��ϰ��ϵ�д�
�����Ŀ
 2C(g)������2s����C��Ũ��Ϊ0.6mol��L-1���������м���˵����
2C(g)������2s����C��Ũ��Ϊ0.6mol��L-1���������м���˵���� NH4++NH2����NH4+��ƽ��Ũ��Ϊ1��10��15mol��L��1������˵���������(�� ��)
NH4++NH2����NH4+��ƽ��Ũ��Ϊ1��10��15mol��L��1������˵���������(�� ��) XC���������� 2 s���룩��Ӧ��ƽ��,��� C ��Ũ��Ϊ 0.6 mol��L��1 ��B�����ʵ���Ϊ1.4 mol,�������м���˵����
XC���������� 2 s���룩��Ӧ��ƽ��,��� C ��Ũ��Ϊ 0.6 mol��L��1 ��B�����ʵ���Ϊ1.4 mol,�������м���˵���� 2C(g)������ӦΪ���ȷ�Ӧ������ȷͼ���ǣ� ��
2C(g)������ӦΪ���ȷ�Ӧ������ȷͼ���ǣ� ��
 C��s����4D��g����Q����ͼ��a��b��ʾһ�������£�D�����������ʱ��t�ı仯�������Ҫʹ����b��Ϊ����a���ɲ�ȡ�Ĵ�ʩ�ǣ�������B��Ũ�� �����߷�Ӧ�¶� ����С��Ӧ�������������ѹ�� �ܼ������( )
C��s����4D��g����Q����ͼ��a��b��ʾһ�������£�D�����������ʱ��t�ı仯�������Ҫʹ����b��Ϊ����a���ɲ�ȡ�Ĵ�ʩ�ǣ�������B��Ũ�� �����߷�Ӧ�¶� ����С��Ӧ�������������ѹ�� �ܼ������( )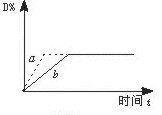
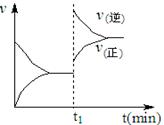
 2SO3 (g) ����H��0
2SO3 (g) ����H��0 B��g��+2C��g��������ѹǿ��Ӧ���ʼӿ죬A��ת���ʼ�С
B��g��+2C��g��������ѹǿ��Ӧ���ʼӿ죬A��ת���ʼ�С ��H2Ӧ�ӵ��ص�________ (��缫����)ͨ�룻����b�缫�ĵ缫��Ӧ����ʽΪ________��
��H2Ӧ�ӵ��ص�________ (��缫����)ͨ�룻����b�缫�ĵ缫��Ӧ����ʽΪ________��
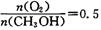 ʱ���Ʊ���Ӧ���������У�����һ����
ʱ���Ʊ���Ӧ���������У�����һ����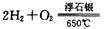
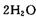 ������һ����____________ (д��ѧ����ʽ����
������һ����____________ (д��ѧ����ʽ����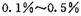 ��HCHO������ɱ����������ԭ����________��
��HCHO������ɱ����������ԭ����________��