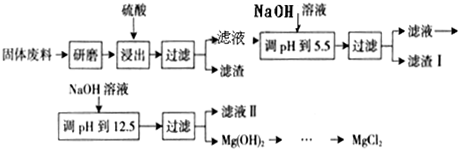
��Ŀ����
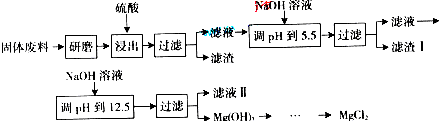
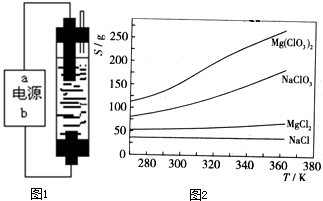
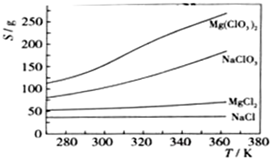
 Mg��ClO3��2��ũҵ�ϳ�������Ҷ������������ɲ��ø��ֽⷴӦ�Ʊ���MgCl2+2NaClO3=Mg��ClO3��2+2NaCl����֪���ֻ�������ܽ�ȣ�S�����¶ȣ�T���仯������ͼ��ʾ������������ȷ����
Mg��ClO3��2��ũҵ�ϳ�������Ҷ������������ɲ��ø��ֽⷴӦ�Ʊ���MgCl2+2NaClO3=Mg��ClO3��2+2NaCl����֪���ֻ�������ܽ�ȣ�S�����¶ȣ�T���仯������ͼ��ʾ������������ȷ����
- A.���ֻ�������ܽ�����¶ȱ仯��С����Mg��ClO3��2
- B.300Kʱ��MgCl2���ܽ��ԼΪ55g
- C.300Kʱ��100gNaCl������Һ��Լ����NaCl 36g
- D.�÷�Ӧ�ܹ����е������������˳������ó�����Mg��ClO3��2
B
������A������ͼ������жϣ�
B������ͼ����Զ����Ȼ�þ���ܽ�ȣ�
C��ͼ������Ȼ����ܽ�����¶ȵı仯�̶Ȳ���ͼ�ж����ܽ����100gˮ�дﵽ����ʱ�ܽ����ʵ�������
D����ͬ�¶�ʱ�Ȼ��Ƶ��ܽ����С���������ȴ��������Mg��ClO3��2������һ���������Ȼ���
���A����ͼ��仯�����õ������ֻ�������ܽ�����¶ȱ仯��С����NaCl����A����
B������ͼ��������Զ�����300Kʱ��MgCl2���ܽ��ԼΪ55g����B��ȷ��
C������ͼ�������300Kʱ��NaCl ���ܽ��ԼΪ36g�������ܽ�ȸ����֪��100gˮ���ܽ�NaCl�ﵽ����ʱ�ܽ��������36g����C����
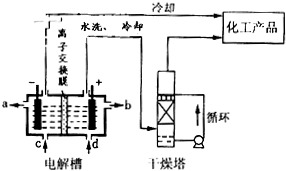
D����ӦMgCl2+2NaClO3�TMg��ClO3��2+2NaCl�����ں�°��Ƽ����NaHCO3��ԭ������ΪNaCl�ܽ��С������Һ��������ʹ��Ӧ������Mg��ClO3��2�ķ�����У���ͬ�¶�ʱ�Ȼ��Ƶ��ܽ����С���������ȴ��������Mg��ClO3��2������һ���������Ȼ��ƣ���D����
��ѡB��
���������⿼���������ܽ�Ⱥ��ܽ�����ߵķ����ж����գ���Ҫ�����ܽ�����¶ȱ仯�ķ�����ͼ�����壬Ӧ�û���֪ʶ�����ѧ����������Լ���ͼ���Ĺ۲졢����������
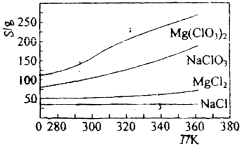
������A������ͼ������жϣ�
B������ͼ����Զ����Ȼ�þ���ܽ�ȣ�
C��ͼ������Ȼ����ܽ�����¶ȵı仯�̶Ȳ���ͼ�ж����ܽ����100gˮ�дﵽ����ʱ�ܽ����ʵ�������
D����ͬ�¶�ʱ�Ȼ��Ƶ��ܽ����С���������ȴ��������Mg��ClO3��2������һ���������Ȼ���
���A����ͼ��仯�����õ������ֻ�������ܽ�����¶ȱ仯��С����NaCl����A����
B������ͼ��������Զ�����300Kʱ��MgCl2���ܽ��ԼΪ55g����B��ȷ��
C������ͼ�������300Kʱ��NaCl ���ܽ��ԼΪ36g�������ܽ�ȸ����֪��100gˮ���ܽ�NaCl�ﵽ����ʱ�ܽ��������36g����C����
D����ӦMgCl2+2NaClO3�TMg��ClO3��2+2NaCl�����ں�°��Ƽ����NaHCO3��ԭ������ΪNaCl�ܽ��С������Һ��������ʹ��Ӧ������Mg��ClO3��2�ķ�����У���ͬ�¶�ʱ�Ȼ��Ƶ��ܽ����С���������ȴ��������Mg��ClO3��2������һ���������Ȼ��ƣ���D����
��ѡB��
���������⿼���������ܽ�Ⱥ��ܽ�����ߵķ����ж����գ���Ҫ�����ܽ�����¶ȱ仯�ķ�����ͼ�����壬Ӧ�û���֪ʶ�����ѧ����������Լ���ͼ���Ĺ۲졢����������

��ϰ��ϵ�д�
�����Ŀ


 Mg��ClO3��2��ũҵ�ϳ�������Ҷ������������ɲ��ø��ֽⷴӦ�Ʊ���MgCl2+2NaClO3=Mg��ClO3��2+2NaCl����֪���ֻ�������ܽ�ȣ�S�����¶ȣ�T���仯������ͼ��ʾ������������ȷ���ǣ�������
Mg��ClO3��2��ũҵ�ϳ�������Ҷ������������ɲ��ø��ֽⷴӦ�Ʊ���MgCl2+2NaClO3=Mg��ClO3��2+2NaCl����֪���ֻ�������ܽ�ȣ�S�����¶ȣ�T���仯������ͼ��ʾ������������ȷ���ǣ�������