��Ŀ����
6���������ȣ�ClO2����һ�ֻ���ɫ�д̼�����ζ�����壬���۵�Ϊ-59��C���е�Ϊ11.0��C��������ˮ����Ŀǰ�����Ϲ��ϵ���һ����Ч�����ס���ȫ��ɱ�������ʼ�����ˮ�����ȷ����й㷺Ӧ�õĸ�Ч��ȫ���������루Cl2����ȣ�ClO2���������и�������ɱ�����������Ҳ��������������DZ��Σ�����л��ȴ��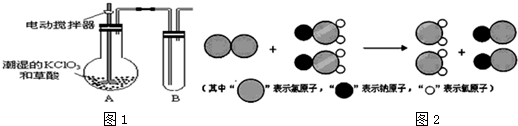
��1����ClO2���Ʊ������У��Ƚ�ʵ�õ�����ʮ�֣�ʵ���ҳ��������ƣ�NaClO3�����������ƣ�Na2SO3�������Ṳ���Ʊ��������ȣ��˻�ѧ��Ӧ����ʽΪ��2NaClO3+Na2SO3+H2SO4=2ClO2��+2Na2SO4+H2O
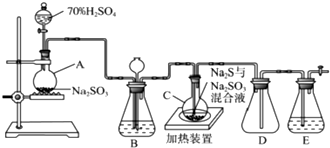
��2����ҵ�����Գ�ʪ��KClO3�Ͳ��ᣨH2C2O4����60��Cʱ��Ӧ�Ƶã�ijѧ������ͼ1��ʾ��װ��ģ�ҵ��ȡ���ռ�ClO2������AΪClO2�ķ���װ�ã�BΪClO2������װ�ã�CΪβ������װ�ã����ʣ�
A���ֻ�Ӧ�����¶ȿ���װ�ã�����˵��һ���¶ȿ��Ʒ���ˮԡ���ȣ�
B���ֻ�Ӧ����ʲôװ�ã���ˮ����������ʵ��װ�û��кβ��㣿ȱ��β������װ��
��3���ҹ�����ɹ����Ƴ���ȡClO2���·������䷴Ӧ���۹�����ͼ2��ʾ���÷�Ӧ�Ļ�ѧ����ʽΪ2NaClO2+Cl2=2NaCl+2ClO2��
���� ��1���������ƣ�NaClO3�����������ƣ�Na2SO3�������Ṳ���Ʊ��������ȣ������������ƺ�ˮ��
��2��ͼ1��ʾ��װ��ģ�ҵ��ȡ���ռ�ClO2������AΪClO2�ķ���װ�ã�BΪClO2������װ�ã�CΪβ������װ�ã�AӦ�����¶�Ϊ60��C��BӦ��ȴ���룬��ʵ����β���ж���
��3�����۹��̿�֪��������NaClO2��Ӧ��
��� �⣺��1���������ƣ�NaClO3�����������ƣ�Na2SO3�������Ṳ���Ʊ��������ȣ������������ƺ�ˮ����ѧ��ӦΪ2NaClO3+Na2SO3+H2SO4=2ClO2��+2Na2SO4+H2O��
�ʴ�Ϊ��2NaClO3+Na2SO3+H2SO4=2ClO2��+2Na2SO4+H2O��
��2��ͼ1��ʾ��װ��ģ�ҵ��ȡ���ռ�ClO2������AΪClO2�ķ���װ�ã�BΪClO2������װ�ã�CΪβ������װ�ã�AӦ�����¶�Ϊ60��C���¶ȿ��Ʒ���Ϊˮԡ���ȣ�BӦ��ȴ���룬B���ֻ�Ӧ�����ˮ����װ�ã���ʵ����β���ж�����ʵ��װ�õIJ���֮��Ϊȱ��β������װ�ã�
�ʴ�Ϊ��ˮԡ���ȣ�����ˮ��������ȱ��β������װ�ã�
��3�����۹��̿�֪��������NaClO2��Ӧ����ԭ�Ӽ������غ��֪����ӦΪ2NaClO2+Cl2=2NaCl+2ClO2���ʴ�Ϊ��2NaClO2+Cl2=2NaCl+2ClO2��
���� ���⿼�����ʵ����ʣ�Ϊ��Ƶ���㣬����ϰ���е���Ϣ��������������ԭ��Ӧ���Ʊ�ʵ��װ�õ����õ�Ϊ���Ĺؼ������ط�����Ӧ�������Ŀ��飬��Ŀ�ѶȲ���

| A�� | ��NaOH��Һ������NH4Cl��KCl | |
| B�� | �������ữ��AgNO3��Һ������NH4Cl��NH4NO3 | |
| C�� | ij��Һ�еμ�BaCl2��Һ�������ɫ�����������Һ��һ������SO42-���� | |
| D�� | ����ɫ��Ӧ����NaCl��KNO3 |
| A�� | �ýྻ�IJ�˿պȡ������Һ�ھƾ��ƻ��������գ�����ʻ�ɫ������Һ��һ��������K+ | |
| B�� | ����һ�����ʵ���Ũ�ȵ���Һʱ������ˮ��������ƿ�Ŀ̶��ߣ��ý�ͷ�ιܽ�����Һ���������� | |
| C�� | �ù�����NaOH��Һ����AlCl3��Һ��MgCl2��Һ | |
| D�� | ����ij��Һ���Ƿ���Fe2+ʱ�����ȼ�����������ˮ���ٵμ����軯����Һ������Һ��Ϊ��ɫ����˵����Һ��һ������Fe2+ |
| A�� | ��ҵ�Ͽ����Ȼ����Ʊ�����NH4Cl | |
| B�� | �ȼҵ�У����۵�����������NaOH | |
| C�� | �ڽӴ��ұ�������SO3��SO3���������ڱ�ˮ�����Ƴ�Ũ���� | |
| D�� | ��ҵ����ʯӢ��̫���ܵ�أ��ڸ����������ȷ������Ʊ��ֲ� |
| Ԫ�� | �й���Ϣ |
| X | Ԫ����Ҫ�������ϼ�Ϊ-2��-1�ۣ�ԭ�Ӱ뾶Ϊ0.074nm�� |
| Y | ��������������������������֮��Ϊ4�� |
| Z | ԭ�Ӱ뾶Ϊ0.102nm����������������������Ӳ�����2��������̬�⻯����Y���ʷ�����Ӧ���ɵ���ɫ���壮 |
| D | ����������Ӧ��ˮ����ܵ����������������������ȵ����������ӣ� |
| E | �����������г�������������Ʒ�ڳ�ʪ�������ױ���ʴ���� |
| F | Ԫ�����ڵ����������������������� |
��1��Z�����ڱ��е�λ�õ������ڵڢ�A�壬D������������Ӧˮ����ĵ���ʽΪ

��2��X������D���ʷ�Ӧ���ɵ�D2X2�ܺ�H2O��Ӧ��д�������ӷ���ʽΪ2Na2O2+2H2O�T4Na++4OH-+O2����
��3��EԪ����YԪ�ؿ��γ�EY2��EY3���ֻ��������۵⻯����Һ�μӼ���EY3��Ũ��Һ��ԭ��ɫ��Һ�ɱ�Ϊ��ɫ����ԭ����2Fe3++2I-�T2Fe2++I2�������ӷ���ʽ��ʾ����
��4��E�����ڷ���������ʴʱ��������Ӧ����ʽO2+2H2O+4e-�T4OH-
��5��F��һ�ָ��γ�������ˮ���������ӷ���ʽ��ʾ�侻ˮԭ��Al3++3H2O�TAl��OH��3�����壩+3H+��
| A�� | ������ڴ���ʯ�ϣ�2H++CO32-�TCO2��+H2O | |
| B�� | ϡ�������ͭƬ�ϣ�Cu+2H+�TCu2++H2�� | |
| C�� | ��NaOH��Һ��ͨ�����CO2��OH-+CO2�THCO3- | |
| D�� | �������������NaHCO3��Һ��Ӧ��Ca2++OH-+HCO3-�TCaCO3��+H2O |
 ijʵ��С����0.50mol/L NaOH��Һ��0.50mol/L������Һ�����к��ȵIJⶨ��
ijʵ��С����0.50mol/L NaOH��Һ��0.50mol/L������Һ�����к��ȵIJⶨ��������0.50mol/L NaOH��Һ
��1����ʵ���д�ԼҪʹ��245mL NaOH��Һ��������Ҫ����NaOH����5.0g��
��2���ӱ���ѡ�����NaOH��������Ҫ�������ǣ�����ĸ����a b e��
| ���� | ������ƽ�������룩 | С�ձ� | ����ǯ | ������ | ҩ�� | ��Ͳ |
| ���� |  |  |  |  |  |  |
| ��� | a | b | c | d | e | f |
��1��д���÷�Ӧ���Ȼ�ѧ����ʽ���к���Ϊ57.3kJ/mol����$\frac{1}{2}$H2SO4��aq��+NaOH��aq��=$\frac{1}{2}$Na2SO4��aq��+H2O��l����H=-57.3kJ/mol��
��2��ȡ50mL NaOH��Һ��30mL������Һ����ʵ�飬ʵ�����������
������д�±��еĿհף�
| �¶� ʵ�� ���� | ��ʼ�¶�t1/�� | ��ֹ�¶� t2/�� | �¶Ȳ�ƽ��ֵ ��t2-t1��/�� | ||
| H2SO4 | NaOH | ƽ��ֵ | |||
| 1 | 26.2 | 26.0 | 26.1 | 30.1 | |
| 2 | 27.0 | 27.4 | 27.2 | 31.3 | |
| 3 | 25.9 | 25.9 | 25.9 | 29.8 | |
| 4 | 26.4 | 26.2 | 26.3 | 30.4 | |
������ʵ����ֵ�����57.3kJ/mol��ƫ�����ƫ���ԭ������ǣ�����ĸ��acd��
a��ʵ��װ�ñ��¡�����Ч����
b����ȡNaOH��Һ�����ʱ���Ӷ���
c���ֶ�ΰ�NaOH��Һ����ʢ�������С�ձ���
d�����¶ȼƲⶨNaOH��Һ��ʼ�¶Ⱥ�ֱ�ӲⶨH2SO4��Һ���¶ȣ�