��Ŀ����
5����֪��25��ʱ��Ksp[Mg�vOH�w2]=3.2��10-11��Ksp[Cu�vOH�w2]�T2.0��10-20��1����25��ʱ����0.02mol/L��MgCl2��Һ�м���NaOH���壬�������Mg��OH��2������Ӧʹ��Һ�е�c��OH-����СΪ4��10-5mol/L��
��2����25��ʱ����Ũ�Ⱦ�Ϊ0.02mol/L��MgCl2��CuCl2�����Һ����μ���NaOH��Һ��������Cu��OH��2�������ѧʽ�������ɸó��������ӷ���ʽΪCu2++2OH-=Cu��OH��2���������ֳ�������ʱ��$\frac{c��M{g}^{2+}��}{c��C{u}^{2+}��}$=1.6��109��
���� ��1������Mg��OH��2������Ӧʹ��Һ�е�c��OH-����СΪ$\sqrt{\frac{Ksp[Mg��OH��_{2}]}{c��M{g}^{2+}��}}$��
��2��KspС���ȳ����������ֳ�������ʱ��$\frac{c��M{g}^{2+}��}{c��C{u}^{2+}��}$=$\frac{c��M{g}^{2+}��{c}^{2}��O{H}^{-}��}{c��C{{u}^{2+}}^{\;}��{c}^{2}��O{{H}^{-}}_{\;}��}$=$\frac{ksp[Mg��OH��_{2}]}{Ksp[Cu��OH��_{2}]}$���Դ������
��� �⣺��1����25��ʱ����0.02mol/L��MgCl2��Һ�м���NaOH���壬�������Mg��OH��2������Ӧʹ��Һ�е�c��OH-����СΪ$\sqrt{\frac{3.2��1{0}^{-11}}{0.02}}$=4��10-5mol/L��
�ʴ�Ϊ��4��10-5��
��2��25��ʱ����Ũ�Ⱦ�Ϊ0.02mol/L��MgCl2��CuCl2�����Һ����μ���NaOH��Һ��KspС���ȳ�������������Cu��OH��2���������ɸó��������ӷ���ʽΪCu2++2OH-=Cu��OH��2���������ֳ�������ʱ��$\frac{c��M{g}^{2+}��}{c��C{u}^{2+}��}$=$\frac{c��M{g}^{2+}��{c}^{2}��O{H}^{-}��}{c��C{{u}^{2+}}^{\;}��{c}^{2}��O{{H}^{-}}_{\;}��}$=$\frac{ksp[Mg��OH��_{2}]}{Ksp[Cu��OH��_{2}]}$=$\frac{3.2��1{0}^{-11}}{2.0��1{0}^{-20}}$=1.6��109��
�ʴ�Ϊ��Cu��OH��2��Cu2++2OH-=Cu��OH��2���� 1.6��109��
���� ���⿼�����ܵ���ʵļ��㣬Ϊ��Ƶ���㣬����Ksp�Ĵ�С��Ksp�ļ��㡢��������Ϊ���Ĺؼ������ط�����Ӧ�������Ŀ��飬ע����������ɼ�KspӦ�ã���Ŀ�ѶȲ���

 �����������Ů��ͯ������ϵ�д�
�����������Ů��ͯ������ϵ�д�| A�� | ���������Ե���� | |
| B�� | ������������ȡƯ�� | |
| C�� | �������������������ά | |
| D�� | �������������������к�θ���ҩ�� |
| A�� | �ڱ���������Ũ��NaCl��Һ | |
| B�� | �ô����������Լ�ƿʢ���� | |
| C�� | ʹ��©������ֽ�����������ձ��Ƚ��й���ʵ�� | |
| D�� | ����100mLŨ��Ϊ0.10 mol•L-1NaCl��Һʱ��������ƿ���ܽ⡢���� |
| A�� | ����̬������ˮ�����磬�۷е�ܵ͵�����һ�����ڷ��Ӿ��� | |
| B�� | ��ȩ����Ȳ�����о��ڦҼ��ͦм�����ÿ�������ЦҼ���Ŀ��ͬ | |
| C�� | ���Ȼ��ס�������̼���������и�ԭ�Ӿ�����8���ӵ��ȶ��ṹ | |
| D�� | HF��HCl��HBr��HI�����ȶ��Ժ����Ծ����μ��� |
2NO��g��+Cl2��g��?2ClNO��g��
��1���о����������������ڴ����к������ӵ������ʱ�������������ȣ��漰���·�Ӧ��
��2NO2��g��+NaCl��s��?NaNO3��s��+ClNO��g����H1 K1
��4NO2��g��+2NaCl��s��?2NaNO3��s��+2Cl2��g��+2NO��g����H2 K2
��2NO��g��+Cl2��g��?2ClNO��g����H3 K3
��H1����H2����H3֮��Ĺ�ϵʽΪ2��H1-��H2��ƽ�ⳣ��K1��K2��K3֮��Ĺ�ϵʽΪK3=$\frac{{{K}_{1}}^{2}}{{K}_{2}}$��
��2����֪���ֻ�ѧ���ļ������������
| ��ѧ�� | NO�еĵ����� | Cl-Cl�� | Cl-N�� | ClNO�е�N=O�� |
| ����/��KJ/mol�� | 630 | 243 | a | 607 |
��3��300��ʱ��2ClNO��g��?2NO��g��+Cl2��g��������Ӧ���ʵı���ʽΪv��=k•cn��ClNO����kΪ���ʳ�����ֻ���¶��йأ���������ϳ���Ũ�ȹ�ϵ�����ʾ��
| ���� | c��ClNO��/��mol/L�� | v/��mol•L•s�� |
| �� | 0.30 | 3.60��10-9 |
| �� | 0.60 | 1.44��10-8 |
| �� | 0.90 | 3.24��10-8 |
��4���������ݻ���Ϊ2L�ĺ����ܱ������зֱ����4mol��2mol ClNO���ڲ�ͬ�¶��·�����Ӧ��2ClNO��g��?2NO��g��+Cl2��g�������ƽ��ʱClNO��Ũ�����¶ȱ仯��������ͼ1��ʾ��ͼ��ABC���λ�������ϣ���

��2ClNO��g��?2NO��g��+Cl2��g����S��0��ѡ�������������=������
��A��B����ƽ�ⳣ��֮��ΪK��A����K��B��=1��27��
��B��C����ClNO��ת����a��B���� a��C�� ��ѡ�������������=������
��5���ڴ���������NO��COת��Ϊ�����壺
2CO��g��+2NO��g��?2CO2��g��+N2��g����H=-748KJ/mol
��һ�������£���λʱ���ڲ�ͬ�¶��²ⶨ�ĵ�������ת������ͼ2��ʾ���¶ȸ���710Kʱ�����¶ȵ����ߵ�������ת���ʽ��͵�ԭ��������¶����ߵ�710Kʱ����λʱ���ڷ�Ӧ��ƽ�⣬�÷�Ӧ�Ƿ��ȷ�Ӧ�������¶ȣ�ƽ�������ƶ���ת���ʽ��ͣ�
����֪���ⶨ������NO��CO�������õ绯ѧ������������������CO�������Ĺ���ԭ����ͼ3��ʾ�������缫�ķ�ӦʽΪCO-2e-+H2O�TCO2+2H+��
| A�� | �������У�﨣�Rb����ȼ�ղ�����Ƶ�ȼ�ղ�������� | |
| B�� | ����At��Ϊ��ɫ���壬AgAt������ˮҲ������ϡ���� | |
| C�� | ��ˮ��Һ�����ԣ�HCl��H2S�����ƶϳ�Ԫ�صķǽ����ԣ�Cl��S | |
| D�� | �衢�λ�ڽ�����ǽ����Ľ��紦�����������뵼����� |

| A�� | ��Ӧ�ڲ��������������зֲ�������ɫ�����²� | |
| B�� | ��Ӧ�ٳ������ܽ��У����л�����Ϊ 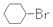 | |
| C�� | ��Ӧ��Ϊ�ӳɷ�Ӧ���������������ױ� | |
| D�� | ��Ӧ����1mol�������3 mol H2�����ӳɷ�Ӧ������Ϊ�����Ӻ�������̼̼˫�� |
 һ��ʯӢ����ľ����ṹ��ͼ��ʾ�����Կ������ھ����ľ�����ÿ������ԭ�Ӽ����һ����ԭ�Ӷ��õ������й�ԭ�ӵ���λ��Ϊ4�����þ����ľ�������Ϊa pm����þ�����ܶ�Ϊ$\frac{480}{{N}_{A}��a��1{0}^{-10}��^{3}}$g/cm3���Ժ�a��NA�Ĵ���ʽ��ʾ����
һ��ʯӢ����ľ����ṹ��ͼ��ʾ�����Կ������ھ����ľ�����ÿ������ԭ�Ӽ����һ����ԭ�Ӷ��õ������й�ԭ�ӵ���λ��Ϊ4�����þ����ľ�������Ϊa pm����þ�����ܶ�Ϊ$\frac{480}{{N}_{A}��a��1{0}^{-10}��^{3}}$g/cm3���Ժ�a��NA�Ĵ���ʽ��ʾ����