��Ŀ����
��26�֣�
������ά�Ļ�ѧ�ɷ��� �����͵���Ҫ�ɷ�������������������˿����ë�Ļ�ѧ�ɷ��� �������������ά����ë?
��һ������50kg�Ľ����ˣ�����Լ����2g������2g���������ڲ����Ե��ʵ���ʽ���ڣ�������Fe2+��Fe3+����ʽ���ڡ��������������ױ����գ���ƶѪ�߲�����ʱ��Ӧ���躬Fe2+�������Σ�����������������ά����C����ʹʳ���е�Fe3+��ԭ��Fe2+���������������ա�
1) �������н���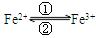 ��ת��ʱ�����е�Fe2+�� �������е�Fe3+�� ��
��ת��ʱ�����е�Fe2+�� �������е�Fe3+�� ��
2) ������ά����C����ʹʳ���е�Fe3+ ��ԭ��Fe2+����仰ָ����ά����C����һ��Ӧ���� �������� �ԣ�
3) �г����۵�ij����Ƭ�к������Ŀ���ϸС�Ļ�ԭ���ۣ���Щ����������θ���������ת���������Ρ��˷�Ӧ�����ӷ���ʽΪ ��
�������Ѿ���Ϊ��Ҫ�Ľ�ͨ���ߣ������ŷŵ�β���ǿ�������Ҫ��Ⱦ��֮һ����֪����β���е���Ҫ��Ⱦ���У�CmHn(��)��SO2��NOX��CO��C�ȣ���ش������й����⡣
1)����CmHn��ʾ���͵���Ҫ��ɣ�CmHn�ڿ�������ȫȼ�յĻ�ѧ����ʽΪ ������ȼ�ղ�������Ϊ�����ṩ�˶�������һ������������ת������ ��ת��Ϊ���ܣ�����ת��Ϊ��е�ܣ�
2)ͨ������ȼ�͵ľ����ӹ��������ɼ�������β���е� (�ѧʽ������ղ��÷�)�ŷţ�
3)Ŀǰ����β������ô�ת���ķ�������������д���ڴ���������NOX��CO��Ӧ�Ļ�ѧ����ʽ ��
������ά�Ļ�ѧ�ɷ��� �����͵���Ҫ�ɷ�������������������˿����ë�Ļ�ѧ�ɷ��� �������������ά����ë?
��һ������50kg�Ľ����ˣ�����Լ����2g������2g���������ڲ����Ե��ʵ���ʽ���ڣ�������Fe2+��Fe3+����ʽ���ڡ��������������ױ����գ���ƶѪ�߲�����ʱ��Ӧ���躬Fe2+�������Σ�����������������ά����C����ʹʳ���е�Fe3+��ԭ��Fe2+���������������ա�
1) ���������
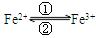 ��ת��ʱ�����е�Fe2+�� �������е�Fe3+�� ��
��ת��ʱ�����е�Fe2+�� �������е�Fe3+�� ��2) ������ά����C����ʹʳ���е�Fe3+ ��ԭ��Fe2+����仰ָ����ά����C����һ��Ӧ���� �������� �ԣ�
3) �г����۵�ij����Ƭ�к������Ŀ���ϸС�Ļ�ԭ���ۣ���Щ����������θ���������ת���������Ρ��˷�Ӧ�����ӷ���ʽΪ ��
�������Ѿ���Ϊ��Ҫ�Ľ�ͨ���ߣ������ŷŵ�β���ǿ�������Ҫ��Ⱦ��֮һ����֪����β���е���Ҫ��Ⱦ���У�CmHn(��)��SO2��NOX��CO��C�ȣ���ش������й����⡣
1)����CmHn��ʾ���͵���Ҫ��ɣ�CmHn�ڿ�������ȫȼ�յĻ�ѧ����ʽΪ ������ȼ�ղ�������Ϊ�����ṩ�˶�������һ������������ת������ ��ת��Ϊ���ܣ�����ת��Ϊ��е�ܣ�
2)ͨ������ȼ�͵ľ����ӹ��������ɼ�������β���е� (�ѧʽ������ղ��÷�)�ŷţ�
3)Ŀǰ����β������ô�ת���ķ�������������д���ڴ���������NOX��CO��Ӧ�Ļ�ѧ����ʽ ��
��������ά�ء�����֬�����������ʡ���ȡ�������ա����ս���ë��ζ������ë
��2��1����ԭ�� ������ 2����ԭ�� ��ԭ�� 3��Fe + 2H+ = Fe2+ +H2 ��
(3) 1) 4CmHn + (4m+n) O2 �� 4m CO2 + 2n H2O ��ѧ��
2��SO2 3) 2NOx + 2xCO �� xN2 + 2x CO2
��2��1����ԭ�� ������ 2����ԭ�� ��ԭ�� 3��Fe + 2H+ = Fe2+ +H2 ��
(3) 1) 4CmHn + (4m+n) O2 �� 4m CO2 + 2n H2O ��ѧ��
2��SO2 3) 2NOx + 2xCO �� xN2 + 2x CO2
��

��ϰ��ϵ�д�
 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
�����Ŀ
 ï��һ�Ƿ�ú��������˹�¹ʣ�Ŀǰ����2����3�ˡ�2�˱�������˹����Ҫ�ɷ��Ǽ��飬�仯ѧʽΪ ���¹ʴ���ר���ڴ�����˹��ը�ֳ�ʱ���������עˮ��עҺ��������������ע��Һ����������ԭ���ǣ� ��
ï��һ�Ƿ�ú��������˹�¹ʣ�Ŀǰ����2����3�ˡ�2�˱�������˹����Ҫ�ɷ��Ǽ��飬�仯ѧʽΪ ���¹ʴ���ר���ڴ�����˹��ը�ֳ�ʱ���������עˮ��עҺ��������������ע��Һ����������ԭ���ǣ� ��

 B�������ʡ���ά�ء����ǡ�PVC�����۶��Ǹ߷��ӻ�����
B�������ʡ���ά�ء����ǡ�PVC�����۶��Ǹ߷��ӻ�����