��Ŀ����
��8�֣�ˮú�������Ǻϳɰ���ԭ����Ҳ�Ǻϳ������仯����Ʒ��ԭ�ϡ�
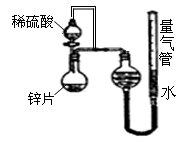
��1��ֱ��ˮú��ȼ�ϵ���У�ͨCO��H2�ļ�Ϊ��ص� ����ѡ�������������������
��2��ˮú���任��Ӧ��CO(g) + H2O(g) CO2(g) + H2(g) ��H �� 0�����д�ʩ����߷�Ӧ���ʵ��� ��������ѡ��
CO2(g) + H2(g) ��H �� 0�����д�ʩ����߷�Ӧ���ʵ��� ��������ѡ��
a.�����¶� b.������� c.����ѹǿ d.����Ũ��
��3��H2��N2�ڴ��������¸�ѹ�����ºϳɰ��Ļ�ѧ����ʽΪ ��
��4��������ˮ��Һ�����������̵����еĶ������÷�Ӧ�Ļ�ѧ����ʽΪ ��
��5������״����582.4L�ϳ�������֪��n(CO)��n(H2)=4��9��ͨ��ϳ�����һ�������¿ɷ���2CO(g)+ 4H2(g) �� CH2=CH2(g)+2H2O(g)��CO(g)+3H2��CH4(g)+H2O(g)����ַ�Ӧ���ⶨ��Ʒ��ֻ�м��顢��ϩ��ˮ�������ٶ�CO��H2����ʣ�ࣩ���Լ����ݳ�����������ϩ�����ʵ������г�������̣���
��1��ֱ��ˮú��ȼ�ϵ���У�ͨCO��H2�ļ�Ϊ��ص� ����ѡ�������������������
��2��ˮú���任��Ӧ��CO(g) + H2O(g)
 CO2(g) + H2(g) ��H �� 0�����д�ʩ����߷�Ӧ���ʵ��� ��������ѡ��
CO2(g) + H2(g) ��H �� 0�����д�ʩ����߷�Ӧ���ʵ��� ��������ѡ��a.�����¶� b.������� c.����ѹǿ d.����Ũ��
��3��H2��N2�ڴ��������¸�ѹ�����ºϳɰ��Ļ�ѧ����ʽΪ ��
��4��������ˮ��Һ�����������̵����еĶ������÷�Ӧ�Ļ�ѧ����ʽΪ ��
��5������״����582.4L�ϳ�������֪��n(CO)��n(H2)=4��9��ͨ��ϳ�����һ�������¿ɷ���2CO(g)+ 4H2(g) �� CH2=CH2(g)+2H2O(g)��CO(g)+3H2��CH4(g)+H2O(g)����ַ�Ӧ���ⶨ��Ʒ��ֻ�м��顢��ϩ��ˮ�������ٶ�CO��H2����ʣ�ࣩ���Լ����ݳ�����������ϩ�����ʵ������г�������̣���
��1���� ��2��abc ��3��N2 + 3H2 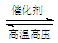 2NH3
2NH3
��4��SO2 + 2NH3 +H2O = (NH4)2SO3 [��SO2 + NH3 +H2O = NH4HSO3]
��5���������⣺n(CO) +n(H2)=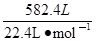 =26mol
=26mol
n(CO)=26mol��4/(4+9)=8mol,n(H2)=26mol��8mol=18mol
�ɷ���ʽ��2CO(g)+ 4H2(g)��C2H4(g)+2H2O(g)��CO(g)+ 3H2(g) �� CH4(g)+ H2O(g)
n(CH4)+2n(C2H4)="8mol" , 3n(CH4)+4n(C2H4)=18mol�����n(C2H4)=3mol��
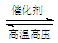 2NH3
2NH3��4��SO2 + 2NH3 +H2O = (NH4)2SO3 [��SO2 + NH3 +H2O = NH4HSO3]
��5���������⣺n(CO) +n(H2)=
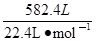 =26mol
=26moln(CO)=26mol��4/(4+9)=8mol,n(H2)=26mol��8mol=18mol
�ɷ���ʽ��2CO(g)+ 4H2(g)��C2H4(g)+2H2O(g)��CO(g)+ 3H2(g) �� CH4(g)+ H2O(g)
n(CH4)+2n(C2H4)="8mol" , 3n(CH4)+4n(C2H4)=18mol�����n(C2H4)=3mol��
�������������ˮú��ȼ�ϵ���У���ԭ��CO��H2����صĸ�����
��ͨ�����¡���ѹ������Ũ�ȡ�����������ܼӿ췴Ӧ���ʣ�
��H2��N2�ڴ��������¸�ѹ�����»��ϳɰ���N2 + 3H2
 2NH3��ע��÷�ӦΪ���淴Ӧ����
2NH3��ע��÷�ӦΪ���淴Ӧ�����Ȱ�����ˮ��Һ���ն���������ܲ���(NH4)2SO3��NH4HSO3��
������ο��𰸡�

��ϰ��ϵ�д�
�����Ŀ
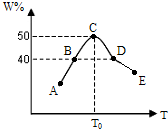
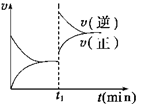
 2C(g) ��H>0
2C(g) ��H>0 xC(g)��D(s)����t2��t3ʱ�̷ֱ�ı䷴Ӧ��һ������(�¶ȡ������������һ������)�����������C(g)��Ũ����ʱ��仯��ͼ��ʾ���й�˵����ȷ����
xC(g)��D(s)����t2��t3ʱ�̷ֱ�ı䷴Ӧ��һ������(�¶ȡ������������һ������)�����������C(g)��Ũ����ʱ��仯��ͼ��ʾ���й�˵����ȷ���� 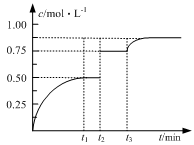
 N2��g��+2CO2��g��������˵������ȷ����
N2��g��+2CO2��g��������˵������ȷ����