��Ŀ����
����Ŀ����֪���ڳ����£�Fe��ˮ������Ӧ�����ڸ����£�Fe��ˮ�����ɷ�����Ӧ��������M���������ƿ���ˮ��Ӧ��������Q������M��������Q��ȼ�ա�
��ʵ��һ��Ӧ����ͼװ�ã���Ӳ�ʲ������з��뻹ԭ���ۺ�ʯ���Ļ����Ϳ�����ɸ����¡�Fe��ˮ�����ķ�Ӧʵ�顱����ش��������⡣

��1��д���÷�Ӧ�Ļ�ѧ����ʽ��_____________________________________��
��2��Բ����ƿ��ʢװˮ���������˼�Ƭ���Ƭ�����Ƭ��������___________��
��3���ƾ��ƺ;ƾ���Ƶ�ȼ��˳�����ȵ�ȼ________________ ��
��ʵ������ں������DZˮͧ�п����ù���������Ϊ��������ij��ѧ̽��ѧϰС��ѡ���ʵ��Ļ�ѧ�Լ���ʵ����Ʒ������ͼʵ��װ�ý���ʵ�飬��֤������������������ʱ����CO2��Ӧ����O2��ͼ�У�A��ʵ�����ô���ʯ��ϡ������ȡCO2��װ�ã�Dװ����װ�й������ƹ��塣
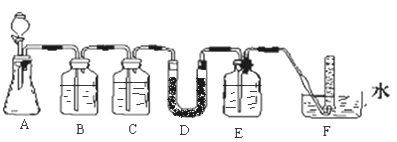
��4��Dװ���з�����Ӧ�Ļ�ѧ����ʽΪ____________________________________��
��5��������ͼ��ʵ��װ����д�±��пո�
���� | �����Լ� | ������Լ���Ŀ�� |
B | ����NaHCO3��Һ | _________________________________�������ӷ���ʽ��ʾ�� |
C | _________________________________ | ��ȥCO2�е�ˮ���� |
��6������Cװ�ã���ʵ�ָ�ʵ��Ŀ���Ƿ���Ӱ�죿_________________________����Ӱ�죬�û�ѧ����ʽ��ʾԭ������Ӱ�죬�����Ӱ�족��
���𰸡�3Fe+ 4H2O��g��![]() Fe3O4+4H2 ��ֹ���� �ƾ��� 2Na2O2+2CO 2=2Na2CO3+O2 HCO3-+H+=H2O+CO2�� Ũ���� 2Na2O2+2H2O=4NaOH+O2
Fe3O4+4H2 ��ֹ���� �ƾ��� 2Na2O2+2CO 2=2Na2CO3+O2 HCO3-+H+=H2O+CO2�� Ũ���� 2Na2O2+2H2O=4NaOH+O2
��������
��1������ˮ��������������ԭ��Ӧ�������������������������ݴ���д��ѧ����ʽ��
��2����ʵ�鰲ȫ�ĽǶȷ������Ƭ�������ǣ�
��3�������һ��װ������Ҫ�������ȣ�һ��Ҫע���ȼ����������˳��һ���Ǹ���ʵ��İ�ȫ�ԺͶ�ʵ������Ӱ�������ǣ�
��4������5������6��ʵ��װ�ÿ�֪����ʵ��������CaCO3�����ᷴӦ����CO2����������ͨ������NaHCO3��Һ���Գ�ȥCO2�����л����HCl������Ũ������������̼��Ȼ�����������CO2��Ӧ������O2���������ˮ���ռ�O2���ݴ˽��
��1������ˮ��������������ԭ��Ӧ������������������������Ӧ�ķ���ʽΪ3Fe+4H2O��g��![]() Fe3O4+4H2��
Fe3O4+4H2��
��2��Բ����ƿ��ʢװˮ���������˼�Ƭ���Ƭ�����ڷ�Ӧ��Ҫ���ȣ�������Ƭ�������Ƿ�ֹ�����¹ʵķ�����
��3����ȼ����������˳��Ҫ����ʵ��İ�ȫ�ԺͶ�ʵ������Ӱ�죬�ڱ�ʵ����Ϊ�˷�ֹ��������е������ڼ�ǿ�ȵ������·�Ӧ��Ӧ���ȵ�ȼ�ƾ��ƣ�����ˮ�������ž�Ӳ�ʲ������ڿ������ٵ�ȼ�ƾ���ƣ�
��4��Dװ���з����ķ�ӦΪ�������ƺͶ�����̼����̼���ƺ���������Ӧ�Ļ�ѧ����ʽΪ2Na2O2+2CO2��2Na2CO3+O2��
��5����������ͨ������NaHCO3��Һ�����Գ�ȥCO2�����л����HCl����Ӧ�����ӷ���ʽΪHCO3-+H+��H2O+CO2����Ȼ������Ũ�����ȥ������̼�е�ˮ��������ֹ���Ź���������CO2��Ӧ��
��6������Cװ�ã�������̼�к���ˮ������ˮ������������Ҳ��Ӧ��������2Na2O2+2H2O��4NaOH+O2������֤���������ƿ���CO2��Ӧ����O2����˶�ʵ�ָ�ʵ��Ŀ����Ӱ�졣





