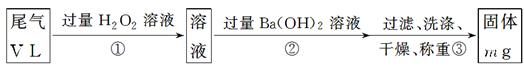��Ŀ����
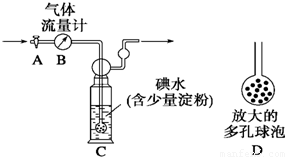
1. (12��)ijУ��ѧʵ����ȤС���ڡ�̽��±�ص��ʵ������ԡ���ϵ��ʵ���з��֣���������ϡ�Ȼ�������Һ�У�����1��2����ˮ������Һ�ʻ�ɫ��
(1)������⣺Fe3����Br2��һ���������Ը�ǿ��
(2)����
�ټ�ͬѧ��Ϊ�����ԣ�Fe3��>Br2��������ʵ�������Ƿ�����ѧ��Ӧ���£�����Һ�ʻ�ɫ�Ǻ�________(�ѧʽ����ͬ)���¡�
����ͬѧ��Ϊ�����ԣ�Br2>Fe3����������ʵ�������Ƿ�����ѧ��Ӧ���£�����Һ�ʻ�ɫ�Ǻ�__________���¡�
(3)���ʵ�鲢��֤
��ͬѧΪ��֤��ͬѧ�Ĺ۵㣬ѡ������ijЩ�Լ���Ƴ����ַ�������ʵ�飬��ͨ���۲�ʵ������֤������ͬѧ�Ĺ۵�ȷʵ����ȷ�ġ���ѡ�õ��Լ���
a����̪��Һ b��CCl4 c����ˮ�ƾ� d��KSCN��Һ
���������б�����д����ͬѧѡ�õ��Լ���ʵ���й۲쵽������(�Լ������)
|
| ѡ���Լ� | ʵ������ |
| ����1 |
|
|
| ����2 |
|
|
(4)Ӧ������չ
����������ϡ�Ȼ�������Һ�м���1��2����ˮ����Һ�ʻ�ɫ�������������ӷ�Ӧ����ʽΪ
_______________________________________________________________________��
����100 mL FeBr2��Һ��ͨ��2.24 L Cl2(��״��)����Һ����1/3��Br���������ɵ���Br2����ԭFeBr2��Һ��FeBr2�����ʵ���Ũ��Ϊ_____________________��
(2)��Br2����Fe3����(3)d����Һ�ʺ�ɫ��b��CCl4�����ɫ��
(4)��2Fe2����Br2===2Fe3����2Br������1.2 mol/L
����:��

 Ʒѧ˫�ž�ϵ�д�
Ʒѧ˫�ž�ϵ�д� Сѧ��ĩ���100��ϵ�д�
Сѧ��ĩ���100��ϵ�д� ��ĩ��ϰ���ϵ�д�
��ĩ��ϰ���ϵ�д��� 12 �֣���1��ijУ��ѧ��ȤС��������ͼ��ʾ���̳�ȥAlCl3�к��е�Mg2����K���������Ӳ������ܼ���AlCl3����ʧ����ش��������⣺
��д��������м�����������������Һʱ����Һ�з�����Ӧ�����ӷ���ʽ��
��
����Һa�д��ڵ���������__________________������Һa�м�������ʱ�������Һ��pH��ԭ����_______________________________________��Ϊ�ˣ��Ľ������� ��
?��2��?�û�ѧС���ֲⶨһ��������ijþ���������þ���������������������ʵ�鷽����
������þ������� �ⶨ������������
�ⶨ������������
������þ������� �ⶨʣ����������
�ⶨʣ����������
�����й��ж��в���ȷ���� ?������ţ�
| A����ҺAѡ��NaOH��Һ |
| B������ҺBѡ��Ũ���ᣬ����þ����������ƫС |
| C����ҺA��B����ѡ��ϡ���� |
| D��ʵ�����з����������ʵʩ |
(12��).��֪�������������Ũ���ᷴӦ�ų�������KClO3 + 6 HCl = KCl + 3Cl2 ��+3H2O��ijУ��ѧ�С���ְ���ͼ����±�ص�����ʵ�顣��������װ�зֱ���в�ͬ��Һ����ɫ����Ӧһ��ʱ��۲����
��1������ͼ��ָ����λ��ɫ�仯Ϊ

|
|
�� |
�� |
�� |
�� |
|
��ɫ |
|
|
|
|
��2��.д���������ӷ�Ӧ����ʽ_____________________________________.
д���������ӷ�Ӧ����ʽ____________________________________________
��3��.���KClO3 + 6 HCl = KCl + 3Cl2 ��+ 3 H2O��Ӧ�е���ת�Ƶķ������Ŀ.

 ���µ����⣬̽��������Э����С���ͬѧ��������о���
���µ����⣬̽��������Э����С���ͬѧ��������о���
 �ܱ�NH3��ԭ��
�ܱ�NH3��ԭ�� ��ɫ������Cu��Ҳ��ͬѧ��ΪNH3��CuO��Ӧ���ɵĺ�ɫ����
��ɫ������Cu��Ҳ��ͬѧ��ΪNH3��CuO��Ӧ���ɵĺ�ɫ���� ��Cu��A�Ļ����������һ����ʵ�����NH3��CuO��Ӧ�����ɵĺ�ɫ�������Ƿ���A�ߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߡ�
��Cu��A�Ļ����������һ����ʵ�����NH3��CuO��Ӧ�����ɵĺ�ɫ�������Ƿ���A�ߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߡ�