��Ŀ����
��20�֣������ݶ����ǵİ�ȫΣ���ܴ���������ԭ�����Լ��˾���Ƿ�ƺ���
2K2Cr2O7(��ɫ)��3C2H5OH��H2SO4 ��Cr2(SO4)3����ɫ����K2SO4��CH3COOH��H2O
����ƽ��ѧ����ʽ��H2Oǰ���ϵ��Ϊ ��
�������ж�˾���Ǿƺ��� ��
��д������ʳ��ƵĻ�ѧ����ʽ�� �� ��
�����п�ͼ�У�A�����ֺ�ɫ��������������ʵ�����϶��ɣ�B�к������������ӡ��ݴ˻ش��������⣺
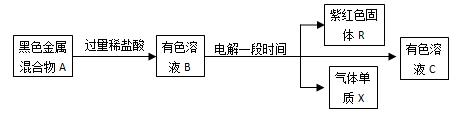
��1��A������� ���ѧʽ����
��2����ͬ�����£���ҺB�����������ӵ���������ǿ������˳�������� ��
��3��A��ij��ֿ��ɵ�����ˮ��Ӧ�Ƶã���ѧ����ʽΪ�� ��
��4���������װ����ͼ��ʾ��
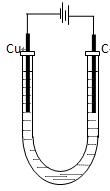
�ٵ�ʼ�Σ������ϵĵ缫��Ӧ�� �������ϵĵ缫��Ӧ�� ��
�ڵ���������տ�ʼ�й���R����ʱ������Һ�н�������Ũ���ɴ�С��˳���� ��
2K2Cr2O7(��ɫ)��3C2H5OH��H2SO4 ��Cr2(SO4)3����ɫ����K2SO4��CH3COOH��H2O
����ƽ��ѧ����ʽ��H2Oǰ���ϵ��Ϊ ��
�������ж�˾���Ǿƺ��� ��
��д������ʳ��ƵĻ�ѧ����ʽ�� �� ��
�����п�ͼ�У�A�����ֺ�ɫ��������������ʵ�����϶��ɣ�B�к������������ӡ��ݴ˻ش��������⣺

��1��A������� ���ѧʽ����
��2����ͬ�����£���ҺB�����������ӵ���������ǿ������˳�������� ��
��3��A��ij��ֿ��ɵ�����ˮ��Ӧ�Ƶã���ѧ����ʽΪ�� ��
��4���������װ����ͼ��ʾ��

�ٵ�ʼ�Σ������ϵĵ缫��Ӧ�� �������ϵĵ缫��Ӧ�� ��
�ڵ���������տ�ʼ�й���R����ʱ������Һ�н�������Ũ���ɴ�С��˳���� ��
��20�֣�ÿ��2�֣�
��11��
�ڽ�˾������������ͨ���ɫ��Һ�У��۲���Һ��ɫ�Ƿ�����ɫת�䡣
�� (C6H10O5)n�� nH2O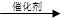 nC6H12O6��C6H12O6
nC6H12O6��C6H12O6 2C2H5OH��2CO2��
2C2H5OH��2CO2��
��1�� Fe3O4 ; CuO ����2�� Fe3�� ;Cu2�� ;H�� ; Fe2����ȫ�Ը��֣���
��3�� 3Fe��4H2O(g) Fe3O4�� 4H2��
Fe3O4�� 4H2��
��4�������� 2Cl���D2e�� = Cl2�������� 2 Fe3����2e�� =" 2" Fe2��������ת�Ʋ��Ȳ����֣���
��Fe2����Cu2������Fe2����Cu2����Fe3����
��11��
�ڽ�˾������������ͨ���ɫ��Һ�У��۲���Һ��ɫ�Ƿ�����ɫת�䡣
�� (C6H10O5)n�� nH2O
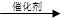 nC6H12O6��C6H12O6
nC6H12O6��C6H12O6 2C2H5OH��2CO2��
2C2H5OH��2CO2����1�� Fe3O4 ; CuO ����2�� Fe3�� ;Cu2�� ;H�� ; Fe2����ȫ�Ը��֣���
��3�� 3Fe��4H2O(g)
 Fe3O4�� 4H2��
Fe3O4�� 4H2����4�������� 2Cl���D2e�� = Cl2�������� 2 Fe3����2e�� =" 2" Fe2��������ת�Ʋ��Ȳ����֣���
��Fe2����Cu2������Fe2����Cu2����Fe3����
��

��ϰ��ϵ�д�
�����Ŀ
 �����������ⶨ�������NaOH�����ʵ��������ó��������ʵ����Ĺ�ϵ����ͼ��ʾ����
�����������ⶨ�������NaOH�����ʵ��������ó��������ʵ����Ĺ�ϵ����ͼ��ʾ����
 ��c��Mg2������c��Al3����Ϊ ��
��c��Mg2������c��Al3����Ϊ �� 2KCl +2MnCl2 +5Cl2��+8H2O��?
2KCl +2MnCl2 +5Cl2��+8H2O��?

 ��2��������һ�����ȼ�50mL8mol/L��HCl��Һ��ʹHCO3����CO32��ȫ����ΪCO2���ټ�50mL2mol/L�� Ba(OH)2��Һ����Ϻ����ҺpH=14����Ϻ���Һ����仯���Բ��ơ��ڳ����£��������A��Na2CO3������Ϊ_______________g��
��2��������һ�����ȼ�50mL8mol/L��HCl��Һ��ʹHCO3����CO32��ȫ����ΪCO2���ټ�50mL2mol/L�� Ba(OH)2��Һ����Ϻ����ҺpH=14����Ϻ���Һ����仯���Բ��ơ��ڳ����£��������A��Na2CO3������Ϊ_______________g�� 2MgO������
2MgO������ NH3�� +CO2�� +H2O��
NH3�� +CO2�� +H2O��  �����ж�Ԫ��As�ļ��鷽���Ͳ��裨As���в�ҩ����Ҫ��As2O3����ʽ���ڣ���
�����ж�Ԫ��As�ļ��鷽���Ͳ��裨As���в�ҩ����Ҫ��As2O3����ʽ���ڣ���