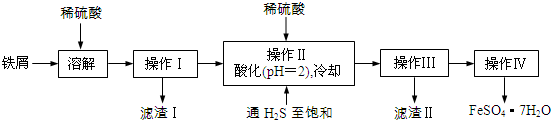
��Ŀ����
1����1.52gͭþ�Ͻ���ȫ�ܽ���50mL�ܶ�Ϊ1.40g•mL-1����������Ϊ63%��Ũ�����У��õ�NO2��N2O4�Ļ������1120mL����״��������Ӧ�����Һ�м���Ũ��Ϊ1.0mol•L-1 ��NaOH��Һ������������ȫ������ʱ���õ�2.54g����������˵������ȷ���ǣ�������| A�� | �úϽ���ͭ��þ������֮����16��3 | |
| B�� | ��Ũ������HNO3�����ʵ���Ũ����14.0 mol•L-1 | |
| C�� | NO2��N2O4�Ļ�������У�N2O4�����������80% | |
| D�� | ��Ӧ��ת�Ƶ��ӵ����ʵ���Ϊ0.06mol |
���� A����������ȫ������ʱ���õ�2.54g����Ϊ������ͭ��������þ���ʳ�����������������Ϊ2.54g-1.52g=1.02g������n=$\frac{m}{M}$���������������ʵ��������ݵ���غ��֪�������ṩ�ĵ������ʵ������������������ʵ��������ݵ���ת���غ����������֮���з��̼�����
B������c=$\frac{1000�Ѧ�}{M}$�����Ũ��������ʵ���Ũ�ȣ�
C������n=$\frac{V}{{V}_{m}}$����NO2��N2O4�����������ʵ�������������������ʵ���Ϊamol�����ݵ���ת���з��̼��㣻
D����������ȫ������ʱ���õ�2.54g����Ϊ������ͭ��������þ���ʳ�����������������Ϊ2.54g-1.52g=1.02g�������������ʵ���Ϊ$\frac{1.02g}{17g/mol}$=0.06mol�����Է�Ӧ��ת�Ƶ��ӵ����ʵ���Ϊ0.06mol��
��� �⣺A����������ȫ������ʱ���õ�2.54g����Ϊ������ͭ��������þ���ʳ�����������������Ϊ2.54g-1.52g=1.02g�������������ʵ���Ϊ$\frac{1.02g}{17g/mol}$=0.06mol�����ݵ���غ��֪�������ṩ�ĵ������ʵ������������������ʵ�������ͭ��þ�Ͻ���Cu��Mg�����ʵ����ֱ�Ϊxmol��ymol����$\left\{\begin{array}{l}{2x+2y=0.06\\;}\\{64x+24y=1.52}\end{array}\right.$�����x=0.02��y=0.01���ʺϽ���ͭ��þ�����ʵ���֮����0.02mol��0.01mol=2��1�����ԸúϽ���ͭ��þ������֮����2��64��1��24=16��3����A��ȷ��
B����Ũ�����ܶ�Ϊ1.40g/mL����������Ϊ63%���ʸ�Ũ��������ʵ���Ũ��Ϊ$\frac{1000��1.4��63%}{63}$mol/L=14mol/L����B��ȷ��
C��NO2��N2O4�����������ʵ���Ϊ$\frac{1.12L}{22.4L/mol}$=0.05mol����������������ʵ���Ϊamol�������������������ʵ���Ϊ��0.05-a��mol�����ݵ���ת���غ��֪��a��1+��0.05-a����2��1=0.06�����a=0.04��NO2�����������$\frac{0.04mol}{0.05mol}$��100%=80%������N2O4�����������1-80%=20%����C����
D����������ȫ������ʱ���õ�2.54g����Ϊ������ͭ��������þ���ʳ�����������������Ϊ2.54g-1.52g=1.02g�������������ʵ���Ϊ$\frac{1.02g}{17g/mol}$=0.06mol�����Է�Ӧ��ת�Ƶ��ӵ����ʵ���Ϊ0.06mol����D��ȷ��
��ѡC��
���� ���⿼��������йؼ��㣬�Ѷ��еȣ����ⷴӦ�����Ĺ����ǹؼ����Ƕ�ѧ���ۺ������Ŀ��飬ע������غ�˼����еĽ��

 �п�������㾫��ϵ�д�
�п�������㾫��ϵ�д�
��֪��MgCl2•6H2O��������Mg��OH�� Cl��HCl����ȣ���������
| A�� | ���̢ٵ��ᴿ�ǻ�ѧ���̣����̢�ͨ��������ԭ��Ӧ�ɲ���2�ֵ��� | |
| B�� | �ڹ��̢��н�MgCl2•6H2Oֱ�����յò���MgCl2 | |
| C�� | �ڹ��̢ܡ���Ӧ��ÿ����0.2 mol Br-������2.24LCl2 | |
| D�� | ���̢ݷ�Ӧ����Һ��ǿ���ԣ���������������豸�ĸ�ʴ���� |
| A�� | ú��������Һ�����ǻ�ѧ�仯 | |
| B�� | BaSO4��ҽѧ���������ͣ�Ba2+�������� | |
| C�� | ��������ͭ�ڿ����г��ڷ��ñ��涼������������ | |
| D�� | ��������ɹ㷺����ʳƷ������ |
| A�� | ��Cl2�е�ȼ��˿����FeCl2���� | |
| B�� | ʵ�������÷�ͭм��ȡCuSO4��Һ����õķ����ǽ�ͭм�ڿ��������պ�������ϡH2SO4 | |
| C�� | С�մ����Ҫ�ɷ���̼���� | |
| D�� | ʵ�������Ȼ����Һ������������Һ�����ȡ���� |
 �����ӡ�ÿ���������桷3•15��Ŀ���������������ࡷ�������������ݵȵ�����������Υ������ҩƷ�����⾫�������������⾫��֮һ�������������ȫ��������ҵ�е�ʹ�ö���Υ���ģ��������FDA�����������˶�Ͱ�����һ�����⾫��������ʹ�ú�������������ʹ��ǰ���С���ҹ�����IJ�𡱣��˿˶�Ͱ���ṹ��ʽ����ͼ�����������в���ȷ���ǣ�������
�����ӡ�ÿ���������桷3•15��Ŀ���������������ࡷ�������������ݵȵ�����������Υ������ҩƷ�����⾫�������������⾫��֮һ�������������ȫ��������ҵ�е�ʹ�ö���Υ���ģ��������FDA�����������˶�Ͱ�����һ�����⾫��������ʹ�ú�������������ʹ��ǰ���С���ҹ�����IJ�𡱣��˿˶�Ͱ���ṹ��ʽ����ͼ�����������в���ȷ���ǣ�������| A�� | C18H23NO3 | |
| B�� | ���˶�����ڷ����廯���� | |
| C�� | ÿĦ���˿˶�Ͱ�����3mol NaOH��Ӧ | |
| D�� | ���˶�Ͱ���ȼ��ȼ��ʱ�����ܲ����ж������������� |
| A�� |  | B�� |  | ||
| C�� |  | D�� |  |