��Ŀ����
(9��)(1)��֪��N N�ļ�����946 kJ��mol��H��H�ļ���Ϊ436 kJ��mol��N��H�ļ���Ϊ391 kJ��mol����ݴ�д���ϳɰ���Ӧ���Ȼ�ѧ����ʽ ��
N�ļ�����946 kJ��mol��H��H�ļ���Ϊ436 kJ��mol��N��H�ļ���Ϊ391 kJ��mol����ݴ�д���ϳɰ���Ӧ���Ȼ�ѧ����ʽ ��
�÷�Ӧ���ر��S 0(�>������<����=��)��
(2)��1 mol N2��3 mol H2��������ݻ�Ϊ10 L���ܱ������С�
���¶�ΪT1ʱ����������а������ʵ�������Ϊ25������N2��ת����Ϊ ��
�ڵ��¶���T1�仯��T2 (T2 >T1)ʱ(��ͼ)��ƽ�ⳣ��KA KB(�>������<����=��)��
���¶���T1ʱ������ʼʱ��10L�ܱ������м���N2 0��5 mol��H2 1��5 mol��NH3 1 mol������Ӧ�ﵽƽ��ʱ������ʼʱ�Ƚϣ� (�ѧʽ)�����ʵ������ӡ�
��1��N2(g) +3H2(g)  2NH3(g) ��H= - 92 kJ/mol ��2�֣� < ��1�֣�
2NH3(g) ��H= - 92 kJ/mol ��2�֣� < ��1�֣�
��2���� 40% ��2�֣� �� > ��2�֣�
��3��N2 ��H2 ��2�֣�
����

��ϰ��ϵ�д�
 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
�����Ŀ
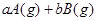

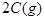
 ���� 1.5 1.0 0
���� 1.5 1.0 0

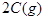
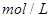 ����
1.5
1.0 0
����
1.5
1.0 0 N�ļ�����946 kJ��mol��H��H�ļ���Ϊ436 kJ��mol��N��H�ļ���Ϊ391 kJ��mol����ݴ�д���ϳɰ���Ӧ���Ȼ�ѧ����ʽ
��
N�ļ�����946 kJ��mol��H��H�ļ���Ϊ436 kJ��mol��N��H�ļ���Ϊ391 kJ��mol����ݴ�д���ϳɰ���Ӧ���Ȼ�ѧ����ʽ
��