��Ŀ����
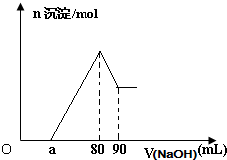 ��һ��þ���Ͻ�Ͷ��һ�������1mol/Lϡ�����У����Ͻ���ȫ�ܽ������Һ�����1mol/L��NaOH��Һ�����ɳ������ʵ��������NaOH��Һ����Ĺ�ϵ��ͼ������˵���в���ȷ����
��һ��þ���Ͻ�Ͷ��һ�������1mol/Lϡ�����У����Ͻ���ȫ�ܽ������Һ�����1mol/L��NaOH��Һ�����ɳ������ʵ��������NaOH��Һ����Ĺ�ϵ��ͼ������˵���в���ȷ����
- A.��ͼ����ȷ����þ���Ͻ�����������Ϊ0.27g
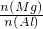
- B.��ͼ����ȷ���úϽ�����Ԫ�����ʵ���֮�ȵ����ֵΪ2.5
- C.������1mol/L��NaOH��Һ85mLʱ�����ó����ijɷ�ΪMg��OH��2��Al��OH��3
- D.��ͼ����ȷ��a��ȡֵ��ΧΪ��0��a��50
D
������A�������������Ƶ������80��90ml���ڣ����е��������������ܽ����������������������Ƶ���ȷ��������������������ԭ���غ�ȷ��������������
B������ͼ�������ĵ��������Ƶ���ȷ���Ͻ��н���þ�������������ȷ���úϽ�����Ԫ�����ʵ���֮�ȵ����ֵ��
C��������1mol/L��NaOH��Һ85mLʱ��ֻ��5ml���������������ܽ⣻
D���Ͻ����ɿ��Բ��ü����跨��������ȫ���ǽ�����ʱʣ�������࣬a��ֵ������ж�a��ȡֵ��Χ��
��𣺸���ͼ��֪�����ȷ����ķ�Ӧ���к������H++OH-=H2O��Ȼ���dz������ֽ������ӣ�Mg2++2OH-=Mg��OH��2����Al3++3OH-=Al��OH��3���������Al��OH��3���ܽ⣺Al��OH��3+OH-=[Al��OH��4]-���Ӻ�����80mL��90mL��ο������n��Al��OH��3��=0.01mol����n��Al��=n��Al��OH��3��=0.01mol����Ϊ0.27g����A��ȷ��
B��n��Al��=n��Al��OH��3��=0.01mol�����Գ���Al3+��Ҫ��OH-Ϊ0.03mol����NaOH��Һ�����Ϊ30mL��þ���Ӻ������ӳ�����ȫ���ĵļ�����Ϊ80-a�����������ʣ�����Գ���Mg2+��ҪNaOH��Һ��������ֵΪ50mL�����n ��Mg�������ֵΪ0.025mol�����ԸúϽ���þ����Ԫ�����ʵ���֮�ȵ����ֵΪ2.5����B��ȷ��
C��������1mol/L��NaOH��Һ85mLʱ��������Ӧ��Al��OH��3+OH-=[Al��OH��4]-��ֻ��5ml���������������ܽ⣬��������ʣ�࣬���ó����ijɷ�ΪMg��OH��2��Al��OH��3����C��ȷ��
D�����������ܽ������ʣ����������£���ǡ����Ͻ�Ӧ��ȫ����a=0��ͨ����ֵ�������Ͻ�����ȫ����ʱ����Ϊ����Al3+��ҪNaOH��Һ�����Ϊ30mL����ͼ��֪���к������������ĵļ�Һ������Ϊ50mL�����Ǽ��費���������ֵ�Dz����ڵģ����Ե�ȡֵ��ΧΪ 0��a��50����D����
��ѡD��
���������⿼��ѧ���йؽ���Ԫ�������仯��������ʵ�֪ʶ�����ͼ����п��飬�������Ѷȣ�
������A�������������Ƶ������80��90ml���ڣ����е��������������ܽ����������������������Ƶ���ȷ��������������������ԭ���غ�ȷ��������������
B������ͼ�������ĵ��������Ƶ���ȷ���Ͻ��н���þ�������������ȷ���úϽ�����Ԫ�����ʵ���֮�ȵ����ֵ��
C��������1mol/L��NaOH��Һ85mLʱ��ֻ��5ml���������������ܽ⣻
D���Ͻ����ɿ��Բ��ü����跨��������ȫ���ǽ�����ʱʣ�������࣬a��ֵ������ж�a��ȡֵ��Χ��
��𣺸���ͼ��֪�����ȷ����ķ�Ӧ���к������H++OH-=H2O��Ȼ���dz������ֽ������ӣ�Mg2++2OH-=Mg��OH��2����Al3++3OH-=Al��OH��3���������Al��OH��3���ܽ⣺Al��OH��3+OH-=[Al��OH��4]-���Ӻ�����80mL��90mL��ο������n��Al��OH��3��=0.01mol����n��Al��=n��Al��OH��3��=0.01mol����Ϊ0.27g����A��ȷ��
B��n��Al��=n��Al��OH��3��=0.01mol�����Գ���Al3+��Ҫ��OH-Ϊ0.03mol����NaOH��Һ�����Ϊ30mL��þ���Ӻ������ӳ�����ȫ���ĵļ�����Ϊ80-a�����������ʣ�����Գ���Mg2+��ҪNaOH��Һ��������ֵΪ50mL�����n ��Mg�������ֵΪ0.025mol�����ԸúϽ���þ����Ԫ�����ʵ���֮�ȵ����ֵΪ2.5����B��ȷ��
C��������1mol/L��NaOH��Һ85mLʱ��������Ӧ��Al��OH��3+OH-=[Al��OH��4]-��ֻ��5ml���������������ܽ⣬��������ʣ�࣬���ó����ijɷ�ΪMg��OH��2��Al��OH��3����C��ȷ��
D�����������ܽ������ʣ����������£���ǡ����Ͻ�Ӧ��ȫ����a=0��ͨ����ֵ�������Ͻ�����ȫ����ʱ����Ϊ����Al3+��ҪNaOH��Һ�����Ϊ30mL����ͼ��֪���к������������ĵļ�Һ������Ϊ50mL�����Ǽ��費���������ֵ�Dz����ڵģ����Ե�ȡֵ��ΧΪ 0��a��50����D����
��ѡD��
���������⿼��ѧ���йؽ���Ԫ�������仯��������ʵ�֪ʶ�����ͼ����п��飬�������Ѷȣ�

��ϰ��ϵ�д�
�����Ŀ
