��Ŀ����
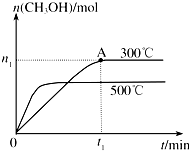 �״���һ�ֿ�����ȼ�ϣ����ݻ�Ϊ2L���ܱ������н��з�Ӧ��CO��g��+2H2��g��?CH3OH��g���������������䣬��300���500��ʱ�����ʵ���n��CH3OH��-��Ӧʱ��t�ı仯������ͼ��ʾ��
�״���һ�ֿ�����ȼ�ϣ����ݻ�Ϊ2L���ܱ������н��з�Ӧ��CO��g��+2H2��g��?CH3OH��g���������������䣬��300���500��ʱ�����ʵ���n��CH3OH��-��Ӧʱ��t�ı仯������ͼ��ʾ����1���÷�Ӧ�ġ�H
��2��300��ʱ��0-t1min�� CH3OH��ƽ����������Ϊ
��3����Ҫ��״��IJ��ʣ��ɲ�ȡ�Ĵ�ʩ��
A����С�������B�������¶�
C�������¶� D��ʹ�ú��ʵĴ���
E���״��ӻ����ϵ�з������
��4��300��ʱ��CO��H2����ʼ���ʵ����ֱ�Ϊ2mol��3mol����ͼ��n1����Ϊ0.5mol���Լ���300���£��÷�Ӧ��ƽ�ⳣ����д��������̣�������λ��Ч���֣�
��5����ҵ��Ҳ������CO2��H2��Ӧ�Ƶü״�����2��105Pa��300��������£�����440g CO2��H2ǡ����ȫ��Ӧ���ɼ״���ˮ���ų�495kJ����������д���÷�Ӧ���Ȼ�ѧ����ʽ
��������1������ͼ�����¶ȶ�ƽ���Ӱ�죬�жϡ�H��
��2������300��ʱ��0-t1min�ڼ״��ı仯�������䷴Ӧ���ʣ�
��3����Ҫ��״��IJ��ʣ�����ƽ�����ƣ�����Ӱ��ƽ������ط�����
��4������ͼ���м״���������������ʽ���ƽ��ʱ�����ʵ�Ũ�ȣ��ٸ���ƽ�ⳣ���ı���ʽ�����
��5�����ݶ�����̼�������ͷų������������1mol������̼��Ӧʱ�ų����������ٸ����Ȼ�ѧ����ʽ����д������д��
��2������300��ʱ��0-t1min�ڼ״��ı仯�������䷴Ӧ���ʣ�
��3����Ҫ��״��IJ��ʣ�����ƽ�����ƣ�����Ӱ��ƽ������ط�����
��4������ͼ���м״���������������ʽ���ƽ��ʱ�����ʵ�Ũ�ȣ��ٸ���ƽ�ⳣ���ı���ʽ�����
��5�����ݶ�����̼�������ͷų������������1mol������̼��Ӧʱ�ų����������ٸ����Ȼ�ѧ����ʽ����д������д��
����⣺��1������ͼ���֪���¶�Խ�ߣ��״������ʵ���ԽС��˵�������¶�ƽ�����ƣ�����������Ϊ���ȷ�Ӧ������H��0���ʴ�Ϊ������
��2����ͼ���֪��300��ʱ0-t1min�ڼ״������ʵ���������n1mol����v��CH3OH��=
=
mol?L-1 min-1���ʴ�Ϊ��
mol?L-1 min-1��
��3����Ҫ��״��IJ��ʣ�����ƽ�����ƣ�
A����С���������ѹǿ������ƽ�����ƣ���A��ȷ��
B���÷�Ӧ������Ϊ���ȷ�Ӧ�������¶ȣ�ƽ�����ƣ���B��ȷ��
C�������¶ȣ�ƽ�����ƣ���C����
D��ʹ�ú��ʵĴ��������淴Ӧ����ͬ�ȳ̶ȵĸı䣬ƽ�ⲻ�ƶ�����D����
E���״��ӻ����ϵ�з��������ƽ�����ƣ���E��ȷ��
�ʴ�Ϊ��ABE��
��4����֪300��ʱ��CO��H2����ʼ���ʵ����ֱ�Ϊ2mol��3mol����ͼ��n1����Ϊ0.5mol������ʼʱ���ʵ���Ũ��c��CO��=1.0mol?L-1��c��H2��=1.5mol?L-1��
ƽ��ʱc��CH3OH��=0.25mol?L-1����
CO��g��+2H2��g��?CH3OH��g��
��ʼ��Ũ�ȣ�mol?L-1����1.0 1.5 0
��Ӧ��Ũ�ȣ�mol?L-1����0.25 0.5 0.25
ƽ���Ũ�ȣ�mol?L-1����0.75 1.0 0.25
��K=
=
��0.33
�𣺸÷�Ӧ��ƽ�ⳣ��0.33��
��5��440g CO2�����ʵ�����
=10mol��10mol������̼�μӷ�Ӧ�ų�495kJ����������1mol������̼�μӷ�Ӧ�ų���������49.5kJ�����Ը÷�Ӧ�Ȼ�ѧ��Ӧ����ʽΪ��CO2��g��+3H2��g��=H2O��g��+CH3OH��g����H=-49.5kJ/mol��
�ʴ�Ϊ��CO2��g��+3H2��g��=H2O��g��+CH3OH��g����H=-49.5kJ/mol��
��2����ͼ���֪��300��ʱ0-t1min�ڼ״������ʵ���������n1mol����v��CH3OH��=
| ||
| t1 |
| n1 |
| 2t1 |
| n1 |
| 2t1 |
��3����Ҫ��״��IJ��ʣ�����ƽ�����ƣ�
A����С���������ѹǿ������ƽ�����ƣ���A��ȷ��
B���÷�Ӧ������Ϊ���ȷ�Ӧ�������¶ȣ�ƽ�����ƣ���B��ȷ��
C�������¶ȣ�ƽ�����ƣ���C����
D��ʹ�ú��ʵĴ��������淴Ӧ����ͬ�ȳ̶ȵĸı䣬ƽ�ⲻ�ƶ�����D����
E���״��ӻ����ϵ�з��������ƽ�����ƣ���E��ȷ��
�ʴ�Ϊ��ABE��
��4����֪300��ʱ��CO��H2����ʼ���ʵ����ֱ�Ϊ2mol��3mol����ͼ��n1����Ϊ0.5mol������ʼʱ���ʵ���Ũ��c��CO��=1.0mol?L-1��c��H2��=1.5mol?L-1��
ƽ��ʱc��CH3OH��=0.25mol?L-1����
CO��g��+2H2��g��?CH3OH��g��
��ʼ��Ũ�ȣ�mol?L-1����1.0 1.5 0
��Ӧ��Ũ�ȣ�mol?L-1����0.25 0.5 0.25
ƽ���Ũ�ȣ�mol?L-1����0.75 1.0 0.25
��K=
| c(CH3OH) |
| c(CO)c2(H2) |
| 0.25 |
| 0.75��12 |
�𣺸÷�Ӧ��ƽ�ⳣ��0.33��
��5��440g CO2�����ʵ�����
| 440g |
| 44g/mol |
�ʴ�Ϊ��CO2��g��+3H2��g��=H2O��g��+CH3OH��g����H=-49.5kJ/mol��
���������⿼���˷�Ӧ���ʵļ��㣬Ӱ��ƽ������أ�ƽ�ⳣ���ļ��㣬�Ȼ�ѧ��Ӧ����ʽ����д���漰��֪ʶ��϶࣬�ۺ��Խ�ǿ���Ѷ��еȣ�

��ϰ��ϵ�д�
�����Ŀ
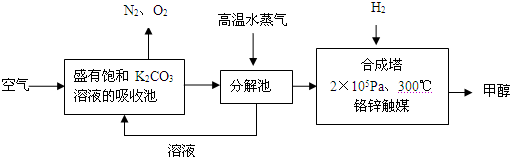
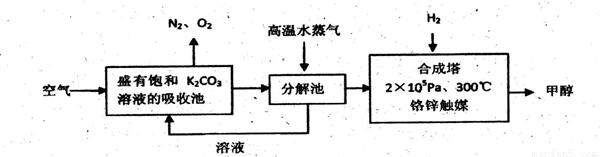
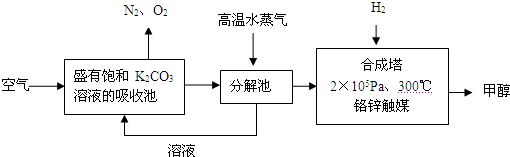
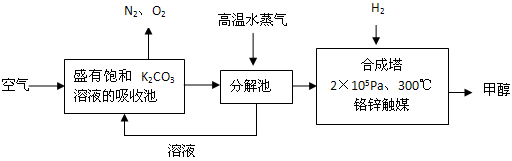
 HCO3�C+ H+��ƽ�ⳣ��K= _________ (����������2λ��Ч���֣���֪10�C5.6=2.5��10�C6)��
HCO3�C+ H+��ƽ�ⳣ��K= _________ (����������2λ��Ч���֣���֪10�C5.6=2.5��10�C6)��