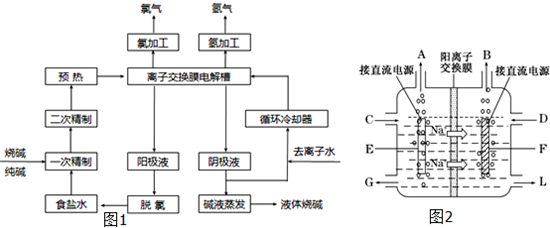
��Ŀ����
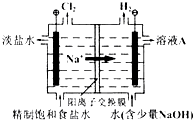 �ȼҵ�е�ⱥ��ʳ��ˮ��ԭ��ʾ��ͼ��ͼ��ʾ��
�ȼҵ�е�ⱥ��ʳ��ˮ��ԭ��ʾ��ͼ��ͼ��ʾ����1����ҺA��������
��2����ⱥ��ʳ��ˮ�����ӷ���ʽ��
��3�����ʱ�����������������Һ��pH��2��3���û�ѧƽ���ƶ�ԭ���������������
��4��������õ���ˮ�辫�ƣ�ȥ����Ӱ���Ca2+��Mg2+��NH4+��SO42-[c��SO42-��c��Ca2+��]�������������£�����ˮ����ҺA���Ե��أ���
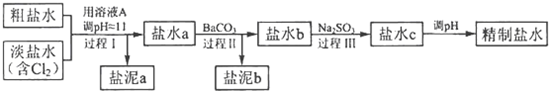
������a����ɳ�⣬�����е�������
�ڹ��̢��н�NH4+ת��ΪN2�����ӷ���ʽ��
��BaSO4���ܽ�ȱ�BaCO3��С�����̢��г�ȥ��������
��������ⱥ��ʳ��ˮʱ��������ӦʽΪ��2H2O-2e-�T2OH-+H2����������ӦʽΪ��2Cl--2e-�TCl2������������ΪNaOH��H2������������Cl2��
��1�����ݵ缫��Ӧ�ж��������
��2�����������ķ�Ӧ��д��ⷴӦʽ��
��3���������������ƽ���ƶ�ԭ��������
��4���ٸ����������Ӻ���Һ��������ж��ܷ�Ӧ���ɵij�����
�ڸ���A��Һ�ɷֺͿ��ܾ��е����ʣ����������ԭ��Ӧ�������غ㶨����д���ӷ���ʽ��
�۸�����Һ�ɷֺ��ܽ�ȴ�С�ж����ɵij�����
��1�����ݵ缫��Ӧ�ж��������
��2�����������ķ�Ӧ��д��ⷴӦʽ��
��3���������������ƽ���ƶ�ԭ��������
��4���ٸ����������Ӻ���Һ��������ж��ܷ�Ӧ���ɵij�����
�ڸ���A��Һ�ɷֺͿ��ܾ��е����ʣ����������ԭ��Ӧ�������غ㶨����д���ӷ���ʽ��
�۸�����Һ�ɷֺ��ܽ�ȴ�С�ж����ɵij�����
����⣺��1����ⱥ��ʳ��ˮʱ��������ӦʽΪ��2H2O-2e-�T2OH-+H2����������ӦʽΪ��2Cl--2e-�TCl2������������ΪNaOH��H2������������Cl2���ݴ˿���ȷ����ҺA��������NaOH���ʴ�Ϊ��NaOH��
��2�������������������ķ�Ӧʽ�ɵõ�ⷴӦ�����ӷ���ʽ��2Cl-+2H2O
H2��+Cl2��+2OH-��
�ʴ�Ϊ��2Cl-+2H2O
H2��+Cl2��+2OH-��
��3�����ʱ�����������������Һ��pH��2��3�������Ǵ�ʹ��ѧƽ��Cl2+H2O HCl+HClO�����ƶ�������Cl2��ˮ�е��ܽ⣬������Cl2���ݳ���
HCl+HClO�����ƶ�������Cl2��ˮ�е��ܽ⣬������Cl2���ݳ���
�ʴ�Ϊ��Cl2��ˮ�ķ�ӦΪCl2+H2O HCl+HClO������HCl��Ũ��ʹƽ�������ƶ�������Cl2��ˮ�е��ܽ⣬������Cl2���ݳ���
HCl+HClO������HCl��Ũ��ʹƽ�������ƶ�������Cl2��ˮ�е��ܽ⣬������Cl2���ݳ���
��4���ٸ��ݴ���ˮ�͵���ˮ�Ļ�ѧ�ɷ֣��������������ˮ�����̽��з�������֪����I�ǽ�Mg2+ת��ΪMg��OH��2������ȥ��������a�г���ɳ�⣬�����е�������Mg��OH��2��
�ʴ�Ϊ��Mg��OH��2��
�ڽ�NH4+ת��ΪN2����������Cl2����Ӧ�����ӷ���ʽ��2NH4++3Cl2+8OH-�TN2��+6Cl-+8H2O��
�ʴ�Ϊ��2NH4++3Cl2+8OH-�TN2��+6Cl-+8H2O��
�۹���II�����ó����ܽ�ƽ��ԭ��������Һ�е�Ca2+��SO42-�ֱ�ת��ΪCaCO3��BaSO4������ȥ��
�ʴ�Ϊ��SO42-��Ca2+��
��2�������������������ķ�Ӧʽ�ɵõ�ⷴӦ�����ӷ���ʽ��2Cl-+2H2O
| ||
�ʴ�Ϊ��2Cl-+2H2O
| ||
��3�����ʱ�����������������Һ��pH��2��3�������Ǵ�ʹ��ѧƽ��Cl2+H2O
 HCl+HClO�����ƶ�������Cl2��ˮ�е��ܽ⣬������Cl2���ݳ���
HCl+HClO�����ƶ�������Cl2��ˮ�е��ܽ⣬������Cl2���ݳ����ʴ�Ϊ��Cl2��ˮ�ķ�ӦΪCl2+H2O
 HCl+HClO������HCl��Ũ��ʹƽ�������ƶ�������Cl2��ˮ�е��ܽ⣬������Cl2���ݳ���
HCl+HClO������HCl��Ũ��ʹƽ�������ƶ�������Cl2��ˮ�е��ܽ⣬������Cl2���ݳ�����4���ٸ��ݴ���ˮ�͵���ˮ�Ļ�ѧ�ɷ֣��������������ˮ�����̽��з�������֪����I�ǽ�Mg2+ת��ΪMg��OH��2������ȥ��������a�г���ɳ�⣬�����е�������Mg��OH��2��
�ʴ�Ϊ��Mg��OH��2��
�ڽ�NH4+ת��ΪN2����������Cl2����Ӧ�����ӷ���ʽ��2NH4++3Cl2+8OH-�TN2��+6Cl-+8H2O��
�ʴ�Ϊ��2NH4++3Cl2+8OH-�TN2��+6Cl-+8H2O��
�۹���II�����ó����ܽ�ƽ��ԭ��������Һ�е�Ca2+��SO42-�ֱ�ת��ΪCaCO3��BaSO4������ȥ��
�ʴ�Ϊ��SO42-��Ca2+��
���������⿼�鱥��ʳ��ˮ�ĵ��ʹ��ε��ᴿ����Ŀ��Ϊ�ۺϣ�ע��ƽ���ƶ�ԭ����Ӧ���Լ��缫��Ӧʽ�����ӷ�Ӧʽ����д������ʱע������������Ϣ�����غ�ķ������

��ϰ��ϵ�д�
�����Ŀ
��ѧ��ҵ�ھ��÷�չ�е����þ������أ������йع�ҵ�����������У���ȷ���ǣ�������
| A�����������г����ø�ѹ�������SO2��ת���� | B���ϳɰ��в��ü�ʱ���백����߷�Ӧ���� | C����⾫��ͭʱ����Һ��c��Cu2+���������ֲ��� | D���ȼҵ��ⱥ��ʳ��ˮʱ�������õ��������ƺ����� |
���в���ȷ���ǣ�������
| A���õ�ⷨ������ͭʱ����ͭ����������ͭ������ | B��п�̸ɵ�ع���ʱ����ص��ܷ�ӦΪ��Zn+2MnO2+2NH4+?Zn2++Mn2O3+2NH3+H2O | C�������绯ѧ��ʴʱ��������ӦʽΪ��2H2O+O2+4e-?4OH- | D���ȼҵ�е�ⱥ��ʳ��ˮ��������ӦʽΪ��2Cl--2e-?Cl2 |
���й��ڡ��ȼҵ����˵���У���ȷ���ǣ�������
| A�����ȼҵ����ԭ����Cl2���ռ� | B�����ȼҵ������Ҫ���������ǵ�ⱥ��ʳ��ˮ | C�����ȼҵ������Ҫ��Ʒ�Ǵ��� | D�����ȼҵ�����ĵ���Դ��Ҫ������ |