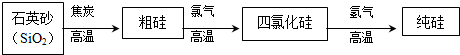
��Ŀ����
ѧϰ��ѧӦ����ȷ����������������������ȥ����������1������д�����ͷ�����Ҫ���εĻ�ѧʽ��
��2������д��θ��ƽ��������������������θ�ᣨ���ᣩ��������ӷ�Ӧ����ʽ��
��3��������KAl��SO4��2?12H2O����һ�־��о�ˮ���õĸ��Σ�����д������ˮ�еĵ��뷽��ʽ��
������������������е���;�� ���еĻ�ѧ���ʽ������飬��������𣺢�Na2CO3 ��NaHCO3 ��Na2O2 ��AgCl ���ƼغϽ� ��SiO2����̫�ռ�DZˮͧ����Ϊ����������
��������1�����ͷ�����Ҫ����Ϊ̼�����ƣ�
��2���������������ᷴӦ�����Ȼ�����ˮ��
��3��KAl��SO4��2Ϊǿ����ʣ�����ȫ���룻
��Na2O2���������̼��Ӧ��������������Ϊ��ֽ���Ʋ�����ԭ�ϵ���Na2CO3���ƼغϽ�������õĵ����ԣ�
��2���������������ᷴӦ�����Ȼ�����ˮ��
��3��KAl��SO4��2Ϊǿ����ʣ�����ȫ���룻
��Na2O2���������̼��Ӧ��������������Ϊ��ֽ���Ʋ�����ԭ�ϵ���Na2CO3���ƼغϽ�������õĵ����ԣ�
����⣺��1�����ͷ�����Ҫ����Ϊ̼�����ƣ���ѧʽΪNaHCO3���ʴ�Ϊ��NaHCO3��
��2���������������ᷴӦ�����Ȼ�����ˮ����Ӧ�����ӷ���ʽΪAl��OH��3+3H+=Al3++3H2O���ʴ�Ϊ��Al��OH��3+3H+=Al3++3H2O��
��3��KAl��SO4��2Ϊǿ����ʣ�����ȫ���룬���뷽��ʽΪKAl��SO4��2=K++Al3++2SO42-��
�ʴ�Ϊ��KAl��SO4��2=K++Al3++2SO42-��
��Na2O2���������̼��Ӧ������������̫�ռ�DZˮͧ����Ϊ������������Ϊ��ֽ���Ʋ�����ԭ�ϵ���Na2CO3���ƼغϽ�������õĵ����ԣ������ڿ����ӷ�Ӧ�����Ƚ�������
�ʴ�Ϊ���ۣ��٣��ݣ�
��2���������������ᷴӦ�����Ȼ�����ˮ����Ӧ�����ӷ���ʽΪAl��OH��3+3H+=Al3++3H2O���ʴ�Ϊ��Al��OH��3+3H+=Al3++3H2O��
��3��KAl��SO4��2Ϊǿ����ʣ�����ȫ���룬���뷽��ʽΪKAl��SO4��2=K++Al3++2SO42-��
�ʴ�Ϊ��KAl��SO4��2=K++Al3++2SO42-��
��Na2O2���������̼��Ӧ������������̫�ռ�DZˮͧ����Ϊ������������Ϊ��ֽ���Ʋ�����ԭ�ϵ���Na2CO3���ƼغϽ�������õĵ����ԣ������ڿ����ӷ�Ӧ�����Ƚ�������
�ʴ�Ϊ���ۣ��٣��ݣ�
���������⿼���Ϊ�ۺϣ��漰Ԫ�ػ�����֪ʶ���ۺ���������ã������ڻ�ѧ����������Ŀ��飬����������ѧ�������õĿ�ѧ���������ѧ����ѧϰ�Ļ����ԣ��ѶȲ���

��ϰ��ϵ�д�
�����Ŀ