��Ŀ����
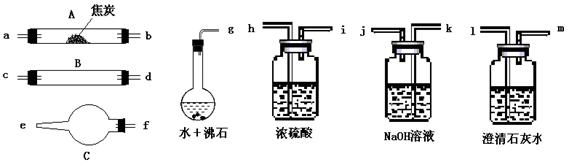
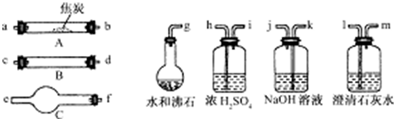
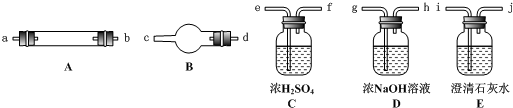
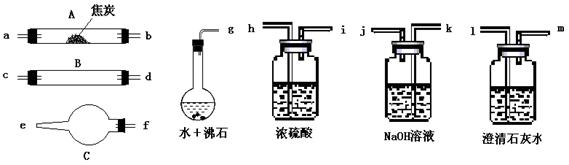
��11�֣�ˮ����ͨ�����Ƚ�̿������������Ҫ��CO��H2������CO2��ˮ�����ȡ�������ͼ��������ѡ���Ҫ���Լ������ʵ��֤�����������������CO��H2��������װ�ú͵��ܵ���ͼ����ȥ��
�� ʢŨ�����װ�õ���;��___________��ʢNaOH��Һ��װ�õ���;��___________��
�� ����B��������Լ��������� �� ����������Ӧ�Ļ�ѧ������
�� �� ��
�� ����C�м��Լ��Ļ�ѧʽ�� ����Ŀ���� ��
�� �������������Ӹ�����������ĸ��ʾ�ӿڵ�����˳��g��ab ��������
�� ��֤��������к���CO��ʵ�������� ��
��֤��������к�H2��ʵ������ ��
��1����ȥˮ����������ȥCO2��������ͭ
��2��CuO+H2=Cu+H2O CuO+CO=Cu+CO2
��3��CuSO4����������H2O
��4���Dkj�Dhi�Dcd(��dc)�Dfe�Dlm
��5��ʯ��ˮ����� ����ˮ����ͭ�ɰױ���
����:��

��ϰ��ϵ�д�
�����Ŀ
 ��������
��������