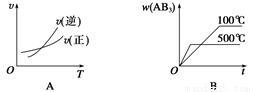��Ŀ����
������ˮ�п��ܴ��ڵ���ƽ�⡢�ε�ˮ��ƽ��ͳ������ܽ�ƽ�⣬���Ƕ��ɿ�����ѧƽ�⡣�������ѧ֪ʶ�ش�
��1����0.1 mol��L��1��(NH4)2SO4��Һ�У��������ӵ�Ũ���ɴ�С˳��Ϊ________��
��2��ʵ����0.1 mol��L��1NaHCO3��Һ��pH��7����ӵ����ˮ���������������NaHCO3��Һ�Լ��Ե�ԭ��_____________________________________�����û�ѧ���P������ֻش�
��3������ͨ����ĭ������ڵ�����Ͳ��ʢ��Al2(SO4)3��Һ����Ͳ��ʢ��NaHCO3��Һ��������ԭ����__________________�����û�ѧ���P������ֻش�
�ڲ��ܰ�Al2(SO4)3��Һʢ������Ͳ�е�ԭ����___________�����û�ѧ���P������ֻش𣩢۲����ܽ�Ƚϴ��Na2CO3����NaHCO3��Һ��ԭ����___________________________��
��ϰ��ϵ�д�
�����Ŀ


 )�뱽��Ϊͬ���칹��
)�뱽��Ϊͬ���칹��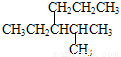 ����ȷ������2-��-3-��������
����ȷ������2-��-3-��������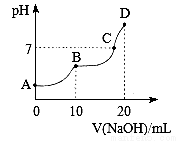

 )��2c(Cl��)
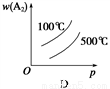
)��2c(Cl��) A2(g)��3B2(g)����H>0������ͼ������ȷ����
A2(g)��3B2(g)����H>0������ͼ������ȷ����