��Ŀ����
 ij������ȤС��Ϊ̽��ij�����Ͻ𣨺Ͻ�Ԫ��ΪMg Al���Ƿ���Ϲ��������������ҹ涨�������������ܵ���78%���������ͼװ�ý���ʵ�飮
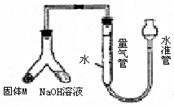
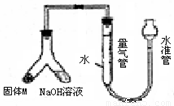
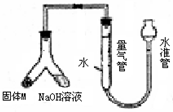
ij������ȤС��Ϊ̽��ij�����Ͻ𣨺Ͻ�Ԫ��ΪMg Al���Ƿ���Ϲ��������������ҹ涨�������������ܵ���78%���������ͼװ�ý���ʵ�飮��1����μ����װ�õ�������
��װ��װ�ò����������ڼ���ˮ�������ƶ�ˮ�ܣ�����Ƭ�̣���ˮ����Һ����������Һ��ά��һ���ĸ߶Ȳ˵��װ���ܷ�
��װ��װ�ò����������ڼ���ˮ�������ƶ�ˮ�ܣ�����Ƭ�̣���ˮ����Һ����������Һ��ά��һ���ĸ߶Ȳ˵��װ���ܷ�
����2���Ͻ���Ʒ����M������������Һ��Ӧ�����ӷ���ʽ
2OH-+2Al+6H2O�T2[Al��OH��4]-+3H2��
2OH-+2Al+6H2O�T2[Al��OH��4]-+3H2��
����3����б���װ��ʹ����������Һ����������ag�Ͻ��ĩ������M����ַ�Ӧ������Ӧֹͣ������������������ΪVmL��������ɱ�״���������������������Ϊ50mL���������M������������mA1����ΧΪ
0��mAl��0.04g
0��mAl��0.04g
����4������װ���е�����������Һ�滻Ϊ���������ᣬ��Ӧֹͣ�����������������
��
��
���������������=����VmL����5����a=38mg��V=44.8mL����״��������ͨ������˵���úϽ��Ƿ���Ϲ��ұ�����д������̣�
����
����
������ϡ������ϡ�������������1������װ�õ������Գ��÷���Ϊ�������������������Һ�������γ�Һ��߶Ȳ��װ��ͼ��֪�ʺ�ѡ��Һ�������γ�Һ��߶Ȳһ��ʱ�䣬Һ��߶Ȳ�ֲ��䣬˵�����������ã�
��2�������������ơ�ˮ��Ӧ�������ǻ��������ơ�������
��3���������������Ϊ50mL��������������������Ϊ50mL������������������ʵ��������ݵ���ת���غ��֪3n��Al��=2n��H2�����ݴ˼����������������
��4��Mg��Al�������ᷴӦ���������������������������������
��5���������������V=44.8mL�������������ʵ��������ݵ���ת���غ��֪3n��Al��=2n��H2�����ݴ˼��������������ټ���Al�������������ݴ��жϣ�
��2�������������ơ�ˮ��Ӧ�������ǻ��������ơ�������
��3���������������Ϊ50mL��������������������Ϊ50mL������������������ʵ��������ݵ���ת���غ��֪3n��Al��=2n��H2�����ݴ˼����������������
��4��Mg��Al�������ᷴӦ���������������������������������
��5���������������V=44.8mL�������������ʵ��������ݵ���ת���غ��֪3n��Al��=2n��H2�����ݴ˼��������������ټ���Al�������������ݴ��жϣ�
����⣺��1������װ�õ������Գ��÷���Ϊ�������������������Һ�������γ�Һ��߶Ȳ��װ��ͼ��֪�ʺ�ѡ��Һ�������γ�Һ��߶Ȳ�����װ�������Եķ���Ϊ����װ��װ�ò����������ڼ���ˮ�������ƶ�ˮ�ܣ�����Ƭ�̣���ˮ����Һ����������Һ��ά��һ���ĸ߶Ȳ˵��װ���ܷ⣬
�ʴ�Ϊ����װ��װ�ò����������ڼ���ˮ�������ƶ�ˮ�ܣ�����Ƭ�̣���ˮ����Һ����������Һ��ά��һ���ĸ߶Ȳ˵��װ���ܷ⣻
��2�������������ơ�ˮ��Ӧ�������ǻ��������ơ���������Ӧ���ӷ���ʽΪ2OH-+2Al+6H2O�T2[Al��OH��4]-+3H2����
�ʴ�Ϊ��2OH-+2Al+6H2O�T2[Al��OH��4]-+3H2����
��3���������������Ϊ50mL��������������������Ϊ50mL������������������ʵ���Ϊ
=
mol�����ݵ���ת���غ��֪3n��Al��=2n��H2��=
mol��2���������������Ϊ
��
mol��2��27g/mol=0.04g�����Խ�������������ΧΪ0��mAl��0.04g��
�ʴ�Ϊ��0��mAl��0.04g��
��4��Mg��Al�������ᷴӦ����������������������������������ʴ�Ϊ������
��5��44.8mL���������ʵ���Ϊ
=0.002mol�����ݵ���ת���غ��֪3n��Al��=2n��H2��=0.002mol��2=0.004mol�����ԺϽ�����������
��0.004mol��27g/mol=0.036g���Ͻ���Al����������Ϊ
��100%=94.74%��78%���ʸúϽ���ϱ����ʴ�Ϊ�����ϣ�
�ʴ�Ϊ����װ��װ�ò����������ڼ���ˮ�������ƶ�ˮ�ܣ�����Ƭ�̣���ˮ����Һ����������Һ��ά��һ���ĸ߶Ȳ˵��װ���ܷ⣻
��2�������������ơ�ˮ��Ӧ�������ǻ��������ơ���������Ӧ���ӷ���ʽΪ2OH-+2Al+6H2O�T2[Al��OH��4]-+3H2����
�ʴ�Ϊ��2OH-+2Al+6H2O�T2[Al��OH��4]-+3H2����
��3���������������Ϊ50mL��������������������Ϊ50mL������������������ʵ���Ϊ
| 0.05L |
| 22.4L/mol |
| 0.05 |
| 22.4 |
| 0.05 |
| 22.4 |
| 1 |
| 3 |
| 0.05 |
| 22.4 |
�ʴ�Ϊ��0��mAl��0.04g��
��4��Mg��Al�������ᷴӦ����������������������������������ʴ�Ϊ������
��5��44.8mL���������ʵ���Ϊ
| 0.0448L |
| 22.4L/mol |
| 1 |
| 3 |
| 0.036g |
| 0.038 |
���������⿼��ѧ����ʵ��ԭ����ʵ��װ�õ����⡢Ԫ�ػ��������ʡ�������ɲⶨ����ѧ����ȣ��Ѷ��еȣ���Ҫѧ���߱���ʵ�Ļ���֪ʶ���ۺ�����֪ʶ�������⡢��������������

��ϰ��ϵ�д�
�����Ŀ
 ij������ȤС��Ϊ̽��ij�����Ͻ𣨺�Mg Al�����������������ͼװ�ý���ʵ�飮
ij������ȤС��Ϊ̽��ij�����Ͻ𣨺�Mg Al�����������������ͼװ�ý���ʵ�飮 ij������ȤС��Ϊ̽��ͭ��Ũ����ķ�Ӧ������������ͼ��ʾװ�ý����й�ʵ�飺
ij������ȤС��Ϊ̽��ͭ��Ũ����ķ�Ӧ������������ͼ��ʾװ�ý����й�ʵ�飺