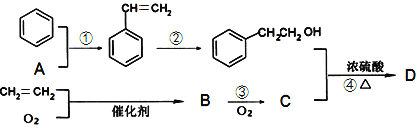
��Ŀ����
����Ŀ����1����֪�������ֵΪ56kJ/g ,д��������ȫȼ�������ȶ������ʵ��Ȼ�ѧ����ʽ��______________________________
��2��һ���¶��£���3 molA�����1mol B����ͨ��һ�ݻ��̶�Ϊ2L���ܱ������У��������·�Ӧ��3A(g)��B(g) ![]() xC(g)����Ӧ1minʱ���ʣ��1.8molA��C��Ũ��Ϊ0.4mol/L����1min�ڣ�B��ƽ����Ӧ����Ϊ______________________��xΪ____________��
xC(g)����Ӧ1minʱ���ʣ��1.8molA��C��Ũ��Ϊ0.4mol/L����1min�ڣ�B��ƽ����Ӧ����Ϊ______________________��xΪ____________��
��3������˵���п��Գ��˵����Ӧ: P��g��+2Q��g��![]() 2R��g��+S��s�����ں��º������Ѵ�ƽ��״̬���� ��__________��
2R��g��+S��s�����ں��º������Ѵ�ƽ��״̬���� ��__________��
A��P��R�������������
B����Ӧ�������ܵ����ʵ������ֲ���
C����Ӧ�����е�ѹǿ����ʱ��ı仯���仯
D����Ӧ������P��Q��R��S���߹���
E. ��Ӧ����Ħ���������ֲ���
��4����ͼΪij�ּ���ȼ�ϵ��ʾ��ͼ������ʱ����������ͼ��ʾ��
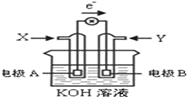
�缫B������___________,����_________��Ӧ��д���缫A�ĵ缫��Ӧʽ��__________���缫A����pH��α仯��_____________________________��
���𰸡�CH4(g)+2O2(g)=CO2(g)+2H2O (l) ��H= -896kJ��mol�C1 0.2mol/(L��min) 2 BCE ���� ��ԭ CH4-8e-+10OH-=CO32-+7H2O ��С
��������
��1����֪�������ֵΪ56kJ/g ,1molCH4������Ϊ16g, ��ȫȼ������CO2��Һ̬H2O���ų�������Ϊ��Q=16g��56kJ/g=896KJ,���Լ�����ȫȼ�������ȶ������ʵ��Ȼ�ѧ����ʽ��CH4(g)+2O2(g)=CO2(g)+2H2O (l) ��H= -896kJ��mol�C1�����Ա���𰸣�CH4(g)+2O2(g)=CO2(g)+2H2O (l) ��H= -896kJ��mol�C1��
��2����������ʽ������ 3A��g��+B��g���TxC��g��
��ʼ��mol/L���� 1.5 0.5 0
ת����mol/L���� 0.6 0.2 0.2x
1min��mol/L�� 0.9 0.3 0.2x
1min�ڣ�B��ƽ����Ӧ����Ϊv=c/t=0.2 molL-1 /1min=0.2mol/(Lmin)
1min��C��Ũ��Ϊ0.4mol/L,���� 0.2x=0.4,x=2�����Ա���𰸣�0.2mol/(Lmin)��2��
��3��A.PΪ��Ӧ�RΪ���P��R������������ȣ�˵�����淴Ӧ���ʲ���ȣ���Ӧδ�ﵽƽ��״̬����A���������⣻
B.�˷�Ӧ�����������С�ķ�Ӧ�������������ʵ������䣬˵��Ũ�Ȳ��ٸı䣬�÷�Ӧ�ﵽƽ��״̬����B�������⡣
C.�˷�Ӧ�����������С�ķ�Ӧ����ѹǿ���淴Ӧ�̶ȷ����仯��˵��Ũ�Ȳ��ٸı䣬�÷�Ӧ�ﵽƽ��״̬����C�������⣻
D.�÷�ӦΪ���淴Ӧ����Ӧ�����У�P��Q��R��S����һֱ���棬���ж��Ƿ�ﵽƽ��״̬����D���������⣻
E. �˷�Ӧ�����������С�ķ�Ӧ������M=m/n��֪����Ӧ����Ħ���������ֲ��䣬˵��Ũ�Ȳ��ٸı䣬�÷�Ӧ�ﵽƽ��״̬����E�������⣻
����������������ȷ��ΪBCE��
��4������ͼ�е��������֪���缫AΪ����������������Ӧ������ȼ�ϵ���е�ȼ�����������缫��ӦʽΪ��CH4-8e-+10OH-=CO32-+7H2O���缫��Ӧ������OH-,���Ըõ缫pH��С���缫BΪ������������ԭ��Ӧ��
���Ա���𰸣����� ����ԭ�� CH4-8e-+10OH-=CO32-+7H2O�� ��С��

 ��ְٷְټ�����Ԫ��ĩ���Ծ�ϵ�д�
��ְٷְټ�����Ԫ��ĩ���Ծ�ϵ�д�