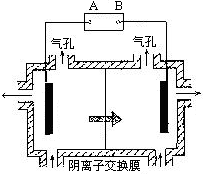
��Ŀ����
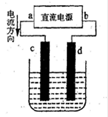
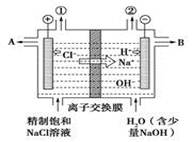
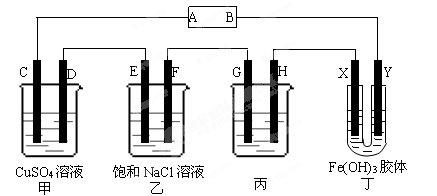
A��B��C����ǿ����ʣ�������ˮ�е����������ΪNa����Ag����NO3-��SO42-��Cl-������ͼ��ʾװ���У��ס��ҡ��������ձ����ηֱ�ʢ��������A��B��C������Һ���缫��Ϊʯī�缫����ͨ��Դ������һ��ʱ�������ձ���c�缫����������10.8�ˡ������¸��ձ�����Һ��pH����ʱ��t�Ĺ�ϵ��ͼ��ʾ���ݴ˻ش��������⣺
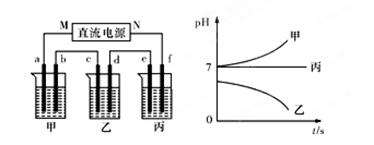
(1)MΪ��Դ���� ����(��д����������)���ס��������ձ��еĵ���ʷֱ�Ϊ���� ���� (��д��ѧʽ)��
(2)����缫f�����ɵ������ڱ�״�������Ϊ���� L��
(3)д�����ձ��еĵ�ⷴӦ����ʽ�� ��
(4)���������Һ�����Ϊ10L�������Һ��pHΪ�� ��

(1)MΪ��Դ���� ����(��д����������)���ס��������ձ��еĵ���ʷֱ�Ϊ���� ���� (��д��ѧʽ)��
(2)����缫f�����ɵ������ڱ�״�������Ϊ���� L��
(3)д�����ձ��еĵ�ⷴӦ����ʽ�� ��
(4)���������Һ�����Ϊ10L�������Һ��pHΪ�� ��
��1���� NaCl AgNO3 ��2��0.56L ��3��4AgNO3��2H2O 4Ag��4HNO3��O2�� ��4��12
4Ag��4HNO3��O2�� ��4��12
 4Ag��4HNO3��O2�� ��4��12
4Ag��4HNO3��O2�� ��4��12�����������1������һ��ʱ�������ձ���c�缫����������10.8�ˣ���˵��c�缫����������M�Ǹ�����N��������f��������e��������d��������b��������a�����������е���pH���ͣ�˵��������ǿ����˵��ҺӦ������������Һ������pH���ߣ�˵��������ǿ����˵��ҺӦ�����Ȼ��ơ�����pH���䣬���൱���ǵ��ˮ����˵��Һ�������ƻ������ơ�
��2��c�缫�����ĵ����������ʵ�����
 ��0.1mol��ת�Ƶ��ӵ����ʵ�����0.1mol��f�缫����������Һ�е�OH���ŵ磬4OH����4e����2H2O��O2��������ݵ���ת���غ��֪���������������ʵ�����
��0.1mol��ת�Ƶ��ӵ����ʵ�����0.1mol��f�缫����������Һ�е�OH���ŵ磬4OH����4e����2H2O��O2��������ݵ���ת���غ��֪���������������ʵ�����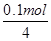 ��0.025mol����״���µ������0.025mol��22.4L/mol��0.56L��
��0.025mol����״���µ������0.025mol��22.4L/mol��0.56L����3�����е��������������Һ�����ⷴӦ����ʽΪ4AgNO3��2H2O
 4Ag��4HNO3��O2����
4Ag��4HNO3��O2������4�����е�������Ȼ��ƣ����ķ���ʽΪ2NaCl��2H2O
 2NaOH��H2����Cl2�������ݵ����غ��֪����Ӧ�������������Ƶ����ʵ�����0.1mol����Ũ����
2NaOH��H2����Cl2�������ݵ����غ��֪����Ӧ�������������Ƶ����ʵ�����0.1mol����Ũ���� ��0.01mol/L����pH��12��
��0.01mol/L����pH��12��
��ϰ��ϵ�д�
 ��У����ϵ�д�
��У����ϵ�д�
�����Ŀ