��Ŀ����
����Ŀ������������һ����ɫ����������ԭ������ҵ���ж��ַ����Ʊ�H2O2��
(1)���о�������H2O2�൱�ڶ�Ԫ���ᣬ������һ�����뷽��ʽΪ_______________����֪������1 L��H2O2�൱��48.3 mol����K1��1.67��10��12������¶���H2O2��c(H��)ԼΪ__________��д����������Ba(OH)2��Ӧ�Ļ�ѧ����ʽ��_____________��
���һ����������Ʊ�����������õķ���������Ҫ���̿�������ͼ��ʾ��д���˹��̵��ܻ�ѧ����ʽ��___________________________________________��

�ۿ����������Ʊ�H2O2��һ�ֻ����Ѻ��͡��������Ʊ�����������ܷ���ʽΪ3H2O��3O2![]() 3H2O2��O3���������ϵ缫��ӦʽΪ________________________��
3H2O2��O3���������ϵ缫��ӦʽΪ________________________��
(2)��Ҳ�����Ԫ���γ�K2O��K2O2��KO2��KO3�����������KO2��һ�ֱ�Na2O2Ч�ʸߵĹ�������д������CO2��Ӧ�Ļ�ѧ����ʽ��__________________________��
���𰸡� H2O2![]() H����HO
H����HO![]() 9��10��6 mol��L��1 Ba(OH)2��H2O2===2H2O��BaO2 H2��O2
9��10��6 mol��L��1 Ba(OH)2��H2O2===2H2O��BaO2 H2��O2![]() H2O2 2H2O��2e��===H2O2��2H�� 4KO2��2CO2===2K2CO3��3O2
H2O2 2H2O��2e��===H2O2��2H�� 4KO2��2CO2===2K2CO3��3O2
��������(1)��˫��ˮ���Կ����Ƕ�Ԫ���ᣬ˵��˫��ˮ�����ܷ����������룬��һ�������һ�������ӣ�������һ�����뷽��ʽΪH2O2H++HO2-������K=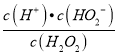 =1.67��10 -12����c(H+)=
=1.67��10 -12����c(H+)=![]() =9��10-6mol/L��H2O2��Ba(OH)2�����γ�����ΪBaO2��ͬʱ����ˮ���䷴Ӧ�ķ���ʽΪ��H2O2+Ba(OH)2=BaO2+2H2O���ʴ�Ϊ��H2O2
=9��10-6mol/L��H2O2��Ba(OH)2�����γ�����ΪBaO2��ͬʱ����ˮ���䷴Ӧ�ķ���ʽΪ��H2O2+Ba(OH)2=BaO2+2H2O���ʴ�Ϊ��H2O2![]() H++HO2-��9��10-6mol/L��H2O2+Ba(OH)2=BaO2+2H2O��
H++HO2-��9��10-6mol/L��H2O2+Ba(OH)2=BaO2+2H2O��
��ͨ��ͼʾ��֪�����һ�������������ȡ˫��ˮ����ӦΪ��H2+O2![]() H2O2���ʴ�Ϊ��H2+O2
H2O2���ʴ�Ϊ��H2+O2![]() H2O2��
H2O2��
�۵���ܷ���ʽ3H2O+3O2![]() 3H2O2+O3��ˮ��������ʧ��������˫��ˮ����缫����ʽ��2H2O-2e-=H2O2+2H+���ʴ�Ϊ��2H2O-2e-=H2O2+2H+��
3H2O2+O3��ˮ��������ʧ��������˫��ˮ����缫����ʽ��2H2O-2e-=H2O2+2H+���ʴ�Ϊ��2H2O-2e-=H2O2+2H+��
(2)�������ƺͶ�����̼��Ӧ����̼���ƺ��������������غͶ�����̼��Ӧ����̼��غ���������Ӧ����ʽΪ4KO2+2CO2=2K2CO3+3O2���ʴ�Ϊ��4KO2+2CO2=2K2CO3+3O2��



