��Ŀ����
ȡһ�����ʵ���Ũ�ȵ�NaOH��Һ100mL��Ȼ������ͨ��һ������CO2���壬�õ���ҺA����A����λ�������0.1mol/L��HCl��Һ��������CO2�����������״����������
HCl��Һ�����֮���ϵ��ͼ��ʾ�������й�˵������ȷ����
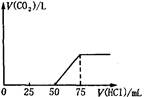
HCl��Һ�����֮���ϵ��ͼ��ʾ�������й�˵������ȷ����

| A��A��Һ������Na2CO3��NaHCO3���ʵ�����Ϊ1��1 |
| B��A��Һ������NaOH��Na2CO3���ʵ�����Ϊ1��1 |
| C��ԭNaOH��Һ���ʵ���Ũ��Ϊ0.075mol/L |
| D��ͨ��CO2�����ڱ���µ����Ϊ56mL |
A
CO2��NaOH��Һ��Ӧ���������֡��߽硱�����
CO2+2NaOH=Na2CO3+H2O����
CO2+NaOH=NaHCO3����
�ʷ�Ӧ����Һ�����ʵijɷֿ������¿��ܣ�Na2CO3��NaOH��Na2CO3��Na2CO3��NaHCO3��NaHCO3��
����ϡ������εε�̼������Һ��ʱ��Na2CO3������ķ�Ӧ�Ƿֲ����еģ�
Na2CO3+HCl=NaHCO3+NaCl����
NaHCO3+HCl=NaCl+H2O+CO2������
���ҿ��Կ���������Ӧ��ȫ����ʱ��������������HCl������ȣ�
����Һ�л����������ƣ������ȷ�����Ӧ��NaOH+HCl=NaCl+H2O����
�跴Ӧ����CO2֮ǰ������������ΪV1���ӿ�ʼ����CO2����Ӧ����������������ΪV2��
�����ַ�Ӧ������ܽ����±���
| ��Һ�����ʵijɷ� | Na2CO3��NaOH | Na2CO3 | Na2CO3��NaHCO3 | NaHCO3 | NaHCO3 |
| ����Һ����μ���ϡ���ᷢ���ķ�Ӧ | �����Ǣݢۢ� | �����Ǣۢ� | �����Ǣۢ� | �� | �� |
| V1��V2�Ĺ�ϵ | V1��V2 | V1=V2 | V1��V2 | V1=0��V2��0 | V1=0��V2��0 |
��1��V1=50mL��V2=75mL-50mL=25mL��V1���ݴ��ж���Һ�����ʵijɷ���Na2CO3��NaOH��
��������ݢۢ�������ѧ����ʽ��֪��NaOH��Na2CO3�����ʵ���֮�ȵ���������������֮�ȣ���
n��NaOH����n��Na2CO3��=��75mL-50mL����25mL=1��1��
��A����ȷ��B��ȷ��
��2������������75mLʱ����Һ����ΪNaCl����ʱn��Na����=n��HCl��=0.075mL��0.1mol��L��1=0.0075mol��
����n��NaOH��=0.0075mol��
c��NaOH��=0.0075mol/0.1L
=0.075mol��L��1��
��C��ȷ��
��3��50mL��75mL�����ķ�ӦΪHCO3��+H��=H2O+CO2����n��HCl��=0.025L��0.1mol��L��1=0.0025mol��
��V��CO2��=0.0025mol��22.4L��mol��1=0.056L=56mL��
��D��ȷ��
ѡA��

��ϰ��ϵ�д�
�����Ŀ
 ��������ȡ�����Ҫװ������:
��������ȡ�����Ҫװ������: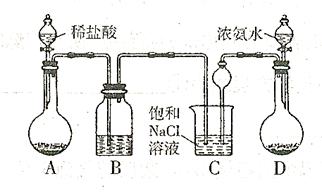
 ,�����D������(ֻ��һ�� ��Cװ��������©���������� ��
,�����D������(ֻ��һ�� ��Cװ��������©���������� �� [
[ �� ��
�� �� Z+ H2O �� Y + 02�� �� X + Ca(OH)2�� Y + CaCO3��
Z+ H2O �� Y + 02�� �� X + Ca(OH)2�� Y + CaCO3�� ol��L��1Na2CO3��Һ�����в�����ȷ���� ( )
ol��L��1Na2CO3��Һ�����в�����ȷ���� ( )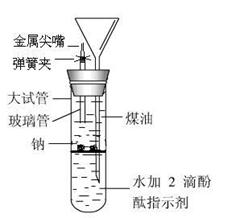
 ��
��