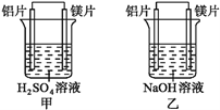
��Ŀ����
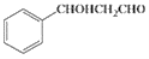
����Ŀ��I������[CO(NH2)2]���˹��ϳɵĵ�һ���л��
��1������������Ԫ�صĵ縺��������________�����ط��Ӽ������ɱ�ʾΪ_____��
��2�����ؿ��������л����ʣ���Ҫ������[Fe(H2NCONH2)6](NO3)3 ��
������ͬ������δ�ɶԵ�������Fe3+��ͬ��Ԫ����________��
��[Fe(H2NCONH2)6](NO3)3���������Ļ�ѧ��____________������ţ���
A ���Ӽ� B ������ C ��λ�� D ���� E ����
��NO3����Nԭ���ӻ���ļ۵����Ų�ͼΪ_______��NO3���Ŀռ乹��Ϊ________��
II��Mg2NiH4��һ������Ľ����⻯��
��3��Mg2NiH4��ͨ���⻯þ����������ĥ�Ƴɡ���Mg2NiH4�����У�Niԭ��ռ����ͼ�Ķ�������ģ�Mg2+����ͼ�˸�С����������ġ�

��Mg2NiH4��H�Ļ��ϼ�Ϊ______��
��Mg2+λ��Niԭ���γɵ�___________�������������϶�������������϶������
����������ܶ�Ϊdg��cm��3��Mg2NiH4��Ħ������ΪMg��mol��1����Mg2+��Niԭ�ӵ���̾���Ϊ___________nm���ú�d��M�Ĵ���ʽ��ʾ����
���𰸡�O N��H��O Mn B 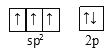 ƽ�������� ��1 �������϶
ƽ�������� ��1 �������϶ ![]() ����
����![]() ��
��
��������
(1)Ԫ�صõ�������Խǿ����縺�Ե���ֵԽ�������X��H��Y��ʾ��
(2)�����ж�Fe3+���е�δ�ɶԵ�������Ȼ��ӵ��������ҳ���Fe3+���е�δ�ɶԵ�����ͬ��Ԫ�أ�
�ڸ���[Fe(H2NCONH2)6](NO3)3�Ľṹ�жϴ��ڵĻ�ѧ�����ͣ�
�۸���NO3-��ԭ�Ӽ��γɵĻ�ѧ���������ж���Nԭ�ӵĺ�������ӻ�������ͣ����ü۲���ӶԻ��������ж���ռ乹�ͣ�
(3)�ٸ��ݻ������к��е�Ԫ�صķǽ����ԣ����Hԭ�ӽṹ����Mg2NiH4��H�Ļ��ϼۣ�
�ڸ������Ŀռ�����ȷ��Mg2+��Niԭ���γɵĿռ乹��ϵ��
�۸����ܶ���=![]() �����㾧���������Ȼ�����V=L3�����㾧��������Mg2+��Niԭ�ӵ���̾���Ϊ������Խ��ߵ�
�����㾧���������Ȼ�����V=L3�����㾧��������Mg2+��Niԭ�ӵ���̾���Ϊ������Խ��ߵ�![]() ���㡣
���㡣
(1) Ԫ�صõ�������Խǿ����縺�Ե���ֵԽ�������صķ����к���C��H��O��N����Ԫ�أ�����Ԫ�صķǽ�����O>N>C>H����֪Ԫ�صĵ縺���ɴ�С��˳��ΪO>N>C>H�����Ե縺������Ԫ����O��
�����ط����У�N��H���Ǽ��Թ��ۼ������õ��Ӷ�ǿ�ҵ�ƫ��Nԭ��һ����ʹHԭ�Ӽ�����Ϊһ����¶�����ӣ�����һ�����ط����е�Oԭ���γ��������ʾΪN��H��O��
(2)��Fe��26��Ԫ�أ�Fe3+������23�����ӣ������Ų�ʽ��1s22s22p63s23p63d5���ɼ�Ϊ�ɶԵ�����Ϊ5��������ԭ�Ӻ�������Ų����ɣ���֪�ڵ���������δ�ɶԵ�������Fe3+һ������25��Ԫ��Mn��
����[Fe(H2NCONH2)6](NO3)3�У�����N-C��N-O��C=O��N=O��N-H���ۼ�����������������˫����һ����������һ�����������ڃȽ�����[Fe(H2NCONH2)6]3+�У��Ƚ�����[Fe(H2NCONH2)6]3+���������NO3-֮��ͨ�����Ӽ���ϣ���������Fe3+����λ��CO(NH2)2֮��ͨ����λ����ϣ������������Ļ�ѧ������������ѡ��B�������⣻
��NO3-����N=O˫�������е�Nԭ�Ӳ���sp2�ӻ����õ����ӻ��������������2s��С��2p���������2�����Ӵ���2p����ϣ�����NO3����Nԭ���ӻ����Nԭ���ӻ���ļ۵����Ų�ͼΪ ��
��
NO3-��Nԭ�ӵŵ��Ӷ���Ϊ![]() =0���۲���Ӷ���Ϊ3+0=3������NO3-�Ŀռ乹��Ϊƽ�������Σ�
=0���۲���Ӷ���Ϊ3+0=3������NO3-�Ŀռ乹��Ϊƽ�������Σ�
(3)���ڻ�����Mg2NiH4�У�ֻ��HԪ��Ϊ�ǽ�����Ԫ�أ���Hԭ�Ӻ���ֻ��1�����ӣ�Ҫ�ﵽK��2�����ӵ��ȶ��ṹ������1�����ӣ�����Mg2NiH4��H�Ļ��ϼ�Ϊ-1�ۣ�
��������Mg2NiH4�����У�Niԭ��ռ����ͼ�Ķ�������ģ�Mg2+����ͼ�˸�С����������ģ�Mg2+����4��Niԭ���γɵ�С������ļ��������ϣ�����������϶��
�۾�����ܶ���=![]() ����һ�������к���Ni��
����һ�������к���Ni��![]() =4������Mg2+��8���ɼ�1�������к���4��Mg2NiH4�����Ը����ܶ���=
=4������Mg2+��8���ɼ�1�������к���4��Mg2NiH4�����Ը����ܶ���=![]() ���ɵ�V=
���ɵ�V= =
=![]() �����Ծ����߳�a=
�����Ծ����߳�a=![]() nm������Mg2+��Niԭ�ӵ���̾���Ϊ������Խ��ߵ�
nm������Mg2+��Niԭ�ӵ���̾���Ϊ������Խ��ߵ�![]() �����Զ��ߵľ���Ϊ
�����Զ��ߵľ���Ϊ![]() nm��
nm��

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�