ЬтФПФкШн
ЁОЬтФПЁПЬњЁЂюмЁЂФјОпгаЯрЫЦЕФаджЪЃЌдкЛЏбЇЩЯГЦЮЊЬњЯЕдЊЫиЁЃЛиД№ЯТСаЮЪЬтЃК
ЃЈ1ЃЉLiCoO2ЁЂ LiFePO4ГЃгУзїяЎРызгЕчГиЕФе§МЋВФСЯЁЃЛљЬЌCoдзгКЫЭтЕчзгХХВМЪНЮЊ_________ЃЌЕкЫФЕчРыФмI4(Co) _________I4(Fe)(ЬюЁА>ЁБЛђЁА<ЁБ)ЃЌPO43ЃЕФПеМфЙЙаЭЮЊ_________ЁЃ
ЃЈ2ЃЉЬњЯЕдЊЫиФмгыCOаЮГЩFe(CO)5ЁЂNi(CO)4ЕШН№ЪєєЪЛљХфКЯЮяЁЃ
гыCOЛЅЮЊЕШЕчзгЬхЕФЗжзгКЭРызгЗжБ№ЮЊ_________КЭ_________(ИїОйвЛжжЃЌЬюЛЏбЇЪН)ЃЛдкCOЗжзгжаЃЌМќгыІаМќЪ§ФПжЎБШЮЊ_________ЁЃ
ЃЈ3ЃЉЬњгыK2OЁЂ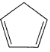 (ЛЗЮьЖўЯЉ)дкИєОјПеЦјЬѕМўЯТЙВШШПЩжЦЕУЖўУЏЬњ[Fe(C5H5)2]ЁЃдкЛЗЮьЖўЯЉжаЃЌCдзгЕФдгЛЏЙьЕРРраЭЮЊ_________ЁЃЖўУЏЬњШлЕуЮЊ446KЃЌВЛШмгкЫЎЃЌвзШмгкввУбЁЂБНЁЂввДМЕШгаЛњШмМСЃЌ373KМДЩ§ЛЊЃЛДгИїжжаджЪПДЃЌЖМБэУїЫќЪЧЕфаЭЕФ_________ЛЏКЯЮяЁЃ
(ЛЗЮьЖўЯЉ)дкИєОјПеЦјЬѕМўЯТЙВШШПЩжЦЕУЖўУЏЬњ[Fe(C5H5)2]ЁЃдкЛЗЮьЖўЯЉжаЃЌCдзгЕФдгЛЏЙьЕРРраЭЮЊ_________ЁЃЖўУЏЬњШлЕуЮЊ446KЃЌВЛШмгкЫЎЃЌвзШмгкввУбЁЂБНЁЂввДМЕШгаЛњШмМСЃЌ373KМДЩ§ЛЊЃЛДгИїжжаджЪПДЃЌЖМБэУїЫќЪЧЕфаЭЕФ_________ЛЏКЯЮяЁЃ
ЃЈ4ЃЉЬњЕЅжЪЕФЖбЛ§ЗНЪНгаСНжжЃЌЦфЦЪУцЭМЗжБ№ШчЭМaЁЂbЫљЪОЁЃ
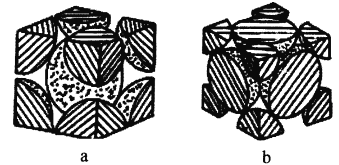
дкЭМaЫљЪОЖбЛ§ЗНЪНРяЬњдзгЕФАыОЖЮЊrpmЃЌдђЦфОЇАћРтГЄЮЊ_________cmЁЃ
дкЭМbЫљЪОЖбЛ§ЗНЪНРяЬњдзгЕФзмЬхЛ§еМОЇЬхЬхЛ§ЕФБШР§ЮЊ_________(гУКЌдВжмТЪІаЕФДњЪ§ЪНБэЪО)
ЁОД№АИЁП[Ar]3d74s2 < е§ЫФУцЬх N2 CN- 1ЃК2 sp2КЭsp3 ЙВМл ![]()
![]() Іа
Іа
ЁОНтЮіЁП
ЃЈ1ЃЉCoЮЊ27КХдЊЫиЃЌдзгКЫЭтЕчзгХХВМЪНЮЊ[Ar]3d74s2ЃЛ
Co3+КЫЭтЕчзгХХВМЮЊВЛЮШЖЈНсЙЙ3d4ЁЂНЯвзЪЇШЅЕчзгЃЌFe3+КЫЭтЕчзгХХВМЮЊАыТњЮШЖЈНсЙЙ3d5,НЯФбЪЇЕчзгЃЌгЩДЫБШНЯЕк4ЕчРыФмЃЛ
PO43-жаPдзгЙТЕчзгЖдЪ§ЮЊ0ЁЂІвМќЪ§ФПЮЊ4ЃЌгЩДЫШЗЖЈПеМфЙЙаЭЃЛ
(2)МлЕчзгЪ§КЭдзгЪ§ЯрЕШЕФСЃзгЛЅЮЊЕШЕчзгЬхЃЛCOгыN2ЕФЙЙЯрЫЦЃЌІвМќЁЂІаМќЪ§ЗжБ№ЮЊ1ЁЂ2ЃЛ
ЃЈ3ЃЉдкЛЗЮьЖўЯЉжага4ИіCдзгЮЊsp2дгЛЏ,1ИіCдзгЮЊsp3дгЛЏЃЛ
ЖўУЏЬњЕФШлЕу,ШмНтадЧщПіАќРЈФмЩ§ЛЊЕШаджЪгыЗжзгОЇЬхЯрЫЦЃЛ
ЃЈ4ЃЉЩшaОЇАћРтГЄЮЊx,4rpm=![]() xЃЌЧѓГіxЃЛЩшbОЇАћРтГЄЮЊyЃЌFeдзгАыОЖЮЊR,4R=
xЃЌЧѓГіxЃЛЩшbОЇАћРтГЄЮЊyЃЌFeдзгАыОЖЮЊR,4R=![]() yЃЌдйНсКЯЧђЬхЛ§ЙЋЪНМЦЫуЁЃ
yЃЌдйНсКЯЧђЬхЛ§ЙЋЪНМЦЫуЁЃ
Нт:ЃЈ1ЃЉCoЮЊ27КХдЊЫи,ЦфМлЕчзгХХВМЮЊ3d74s2,ЛљЬЌCoдзгКЫЭтЕчзгХХВМЪНЮЊ[Ar]3d74s2ЃЛ
Co3+КЫЭтЕчзгХХВМЮЊВЛЮШЖЈНсЙЙ3d4ЁЂНЯвзЪЇШЅЕчзгЃЌFe3+КЫЭтЕчзгХХВМЮЊАыТњЮШЖЈНсЙЙ3d5,НЯФбЪЇЕчзг,ЙЪl4(C0)<l4(Fe)ЃЛ
PO43-жаPдзгЙТЕчзгЖдЪ§ЮЊ0ЁЂІФМќЪ§ФПЮЊ4ЃЌЙЪЦфПеМфЙЙаЭЮЊе§ЫФУцЬхЃЛ
ЃЈ2ЃЉгыCOМлЕчзгЪ§КЭдзгЪ§ЯрЕШЕФЗжзггаN2,РызггаCN-,C22-ЕШЃЛCOгыN2ЕФЙЙЯрЫЦ,ІвМќЁЂІаМќЪ§ЗжБ№ЮЊ1ЁЂ2ЃЌІвМќгыІаМќЪ§ФПжЎБШЮЊ1ЃК2ЃЛ
ЃЈ3ЃЉдкЛЗЮьЖўЯЉжага4ИіCдзгЮЊsp2дгЛЏ,1ИіCдзгЮЊsp3дгЛЏЃЌЖўУЏЬњЕФШлЕуЁЂШмНтадЧщПіАќРЈФмЩ§ЛЊЕШаджЪЃЌЖМБэУїЫќЪЧЕфаЭЕФЙВМлЛЏКЯЮяЃЛ
(4)ЩшaОЇАћРтГЄЮЊx,4rpm=![]() xМДx=
xМДx=![]() rpm=
rpm=![]() rЁС10-10cmЃЌ
rЁС10-10cmЃЌ
ЩшbОЇАћРтГЄЮЊyЃЌFeдзгАыОЖЮЊR,4R=![]() yЃЌМДR=
yЃЌМДR=![]() yЃЌОЇАћКЌгаЕФдзгЪ§ЮЊ(8ЁС1/8+6ЁС1/2)=4,дзгзмЬхЛ§ЮЊ
yЃЌОЇАћКЌгаЕФдзгЪ§ЮЊ(8ЁС1/8+6ЁС1/2)=4,дзгзмЬхЛ§ЮЊ![]() =
=![]() Іаy3,ЬњдзгЕФзмЬхЛ§еМОЇЬхЬхЛ§ЕФБШР§ЮЊ
Іаy3,ЬњдзгЕФзмЬхЛ§еМОЇЬхЬхЛ§ЕФБШР§ЮЊ![]() ІаЁЃ
ІаЁЃ

ЁОЬтФПЁПXЁЂYЁЂZЁЂWЫФжжЖЬжмЦкдЊЫиЃЌгаЙиЪ§ОнШчЯТБэЃЌЯТСаа№Ъіе§ШЗЕФЪЧЃЈ ЃЉ
дЊЫиДњКХ | X | Y | Z | W |
дзгАыОЖ/pm | 66 | 70 | 143 | 160 |
жївЊЛЏКЯМл | -2 | +5ЁЂ+3ЁЂ-3 | +3 | +2 |
A. WКЭYаЮГЩЕФЛЏКЯЮяЮЊЙВМлЛЏКЯЮя
B. YЕФзюИпМлбѕЛЏЮяЖдгІЕФЫЎЛЏЮяЮЊЧПЫс
C. YЕФМђЕЅЦјЬЌЧтЛЏЮяЕФЗаЕуИпгкXЕФМђЕЅЦјЬЌЧтЛЏЮя
D. ЙЄвЕЩЯОГЃВЩгУЕчНтZЕФТШЛЏЮяШмвКжЦБИZЕЅжЪ