��Ŀ����
��16�֣�
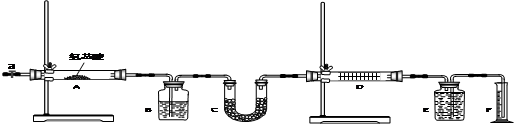
ʵ������ȼ�շ��ⶨ�����谷��CxHyNp���ķ�����ɣ�ȡһ�������������谷���ڴ����г��ȼ�գ�����CO2��H2O��N2��ʵ��װ������29ͼ��ʾ������ÿ������ȫ��Ӧ��
��ش��������⣺
��1��װ�õ�����˳���� �� �� �� �� ������ĸ����
��2��װ��C��ʢװ���Լ��� ��
��3��ʵ�鿪ʼʱ�����ȴ�ֹˮ��a���ر�ֹˮ��b��ͨһ��ʱ��Ĵ�������������Ŀ����
��
��4������װ������Ҫ���ȵ��У���װ����ĸ���ţ� ������ʱӦ�ȵ�ȼ ���ľƾ���.
��5��װ��A�������� ��
��6��ʵ���в�õ�������������㵽����£�������CO2��������ˮ��������Ϊ��ȷ�������谷�ķ���ʽ������Ҫ�������� ��
��1��BCEAD ��4�֣�
��2��ŨH2SO4��2�֣�
��3�����������װ���еĿ�������Ҫ��N2����ʵ��������Ӱ�죨2�֣�
��4��AB��2�֣���A��2�֣�
��5����ȥδ��Ӧ���������2�֣�
��6�������谷��Ħ��������2�֣�
����