��Ŀ����
�¶�t��ʱ��ijNaOHϡ��Һ��c��H+��=10-amol/L��c��OH-��=10-bmol/L����֪��a+b=12����ش��������⣺
��1�����¶���ˮ�����ӻ�����KW=
��2����NaOH��Һ��NaOH�����ʵ���Ũ��Ϊ
��3������NaOH��Һ���ȣ�pH
��1�����¶���ˮ�����ӻ�����KW=
10-12
10-12
����2����NaOH��Һ��NaOH�����ʵ���Ũ��Ϊ
10-bmol/L
10-bmol/L
����NaOH��Һ����ˮ�������c��OH-��Ϊ10-amol/L
10-amol/L
����3������NaOH��Һ���ȣ�pH
��С
��С
����������С�����䡱������������1����Һ�е����ӻ�Kw=C��H+����c��OH-��=10-a��10-b=10-��a+b��=10-12��
��2����NaOH��Һ��NaOH�����ʵ���Ũ��ΪC��NaOH��=c��OH-��=10-bmol/L��������Һ�е����ӻ�Kw=C��H+����c��OH-��=10-12����NaOH��Һ����ˮ�������c��OH-��Ϊ10-a��
��3������NaOH��Һ���ȣ��ٽ�ˮ�ĵ��룬��Һ�д������ӻ����������ӻ�����������Һ������������Ũ���Ǽ������������Ũ�Ȳ��䣬������Ũ������������ҺPH��С��
��2����NaOH��Һ��NaOH�����ʵ���Ũ��ΪC��NaOH��=c��OH-��=10-bmol/L��������Һ�е����ӻ�Kw=C��H+����c��OH-��=10-12����NaOH��Һ����ˮ�������c��OH-��Ϊ10-a��
��3������NaOH��Һ���ȣ��ٽ�ˮ�ĵ��룬��Һ�д������ӻ����������ӻ�����������Һ������������Ũ���Ǽ������������Ũ�Ȳ��䣬������Ũ������������ҺPH��С��
����⣺��1����Һ�е����ӻ�Kw=C��H+����c��OH-��=10-a��10-b=10-��a+b��=10-12���ʴ�Ϊ��10-12��
��2����NaOH��Һ��NaOH�����ʵ���Ũ��ΪC��NaOH��=c��OH-��=10-bmol/L��������Һ�е����ӻ�Kw=C��H+����c��OH-��=10-12����NaOH��Һ����ˮ�������c��OH-��Ϊ10-a��
�ʴ�Ϊ��10-bmol/L��10-amol/L��
��3������NaOH��Һ���ȣ��ٽ�ˮ�ĵ��룬��Һ�д������ӻ����������ӻ�����������Һ������������Ũ���Ǽ������������Ũ�Ȳ��䣬������Ũ������������ҺPH��С��
�ʴ�Ϊ����С��
��2����NaOH��Һ��NaOH�����ʵ���Ũ��ΪC��NaOH��=c��OH-��=10-bmol/L��������Һ�е����ӻ�Kw=C��H+����c��OH-��=10-12����NaOH��Һ����ˮ�������c��OH-��Ϊ10-a��
�ʴ�Ϊ��10-bmol/L��10-amol/L��
��3������NaOH��Һ���ȣ��ٽ�ˮ�ĵ��룬��Һ�д������ӻ����������ӻ�����������Һ������������Ũ���Ǽ������������Ũ�Ȳ��䣬������Ũ������������ҺPH��С��
�ʴ�Ϊ����С��
���������⿼����ˮ��Һ�е����ӻ�����Ӧ�ã���ҺPH����Ӧ�ã��ؼ������ӻ��������¶ȱ仯����Ŀ�ϼ�

��ϰ��ϵ�д�
�����Ŀ
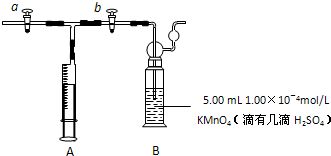
 ��2011?ɽ��ģ�⣩��ȩ����Ҫ���л��ϳ�ԭ�ϣ���������������֬���ϳ���ά��ҩ�Ϳ�ϵȣ�Ҳ������������֯��WHO��ȷ�ϵ��°�����»�����֮һ�����й����ڻ�������ίԱ�����ͳ�ƣ��й���װ��ͥ��ȩ����60%���ϣ��ҹ��涨���ڿ����м�ȩ�������ó���0.08mg/m3��
��2011?ɽ��ģ�⣩��ȩ����Ҫ���л��ϳ�ԭ�ϣ���������������֬���ϳ���ά��ҩ�Ϳ�ϵȣ�Ҳ������������֯��WHO��ȷ�ϵ��°�����»�����֮һ�����й����ڻ�������ίԱ�����ͳ�ƣ��й���װ��ͥ��ȩ����60%���ϣ��ҹ��涨���ڿ����м�ȩ�������ó���0.08mg/m3��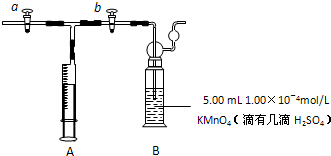
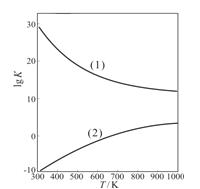

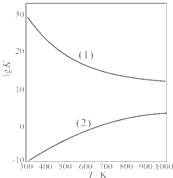
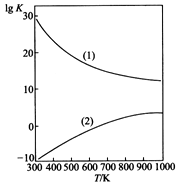
 O2��g����HCHO��g��+H2O��g����H2=-149.73kJ/mol��K2=4.35×1029
O2��g����HCHO��g��+H2O��g����H2=-149.73kJ/mol��K2=4.35×1029
