��Ŀ����
����Ŀ��5�ֹ�������A��B��C��D��E���±��в�ͬ������������ɣ����Ǿ�������ˮ��
������ | Na+�� Al3+ Fe3+�� Cu2+����Ba2+ |
������ | OH����Cl����CO32����NO3����SO4�� |
�ֱ�ȡ���ǵ�ˮ��Һ����ʵ�飬������£�
��A��Һ��C��Һ��Ϻ������ɫ��������ó����м�������ϡHNO3�����������ܽ⣬ʣ���ɫ���壻
��B��Һ��E��Һ��Ϻ�������ɫ������ͬʱ�����������壻
������C��Һ��D��Һ��Ϻ������ɫ����������C��Һ��D��Һ��Ϻ�������
��B��Һ��D��Һ��Ϻ�������
�ݽ�38.4 g CuƬͶ��װ������D��Һ���Թ��У�CuƬ���ܽ⣬�ٵμ�1.6 mol��L��1ϡH2SO4��Cu���ܽ⣬�ܿڸ����к���ɫ������֡�
��1���ݴ��ƶ�A��C�Ļ�ѧʽΪ��A______________��C______________��
��2��д��������з�����Ӧ�Ļ�ѧ����ʽ____________________________________��
��3��D��Һ�е���ʯ����Һ��������___________________________________________��ԭ����_____________________________________________(�����ӷ���ʽ˵��)��
��4�����������Ҫ��CuƬ��ȫ�ܽ⣬���ټ���ϡH2SO4�������____________mL��
��5������500 mL 3 mol��L��1��E��Һ�������11.2 L CO2����(��״�� ��)����Ӧ����Һ�и����ӵ�������Ũ����С�����˳��Ϊ_________________________________��
��6�����ö��Ե缫���A��B�Ļ����Һ�����ʵ����ʵ�����Ϊ0.1 mol����������ϵ�л���ͨ�������������������(��״����)V��ͨ�����ӵ����ʵ���n�Ĺ�ϵ(��������������ˮ)��_________
 ����
����
���𰸡���1��CuSO4Ba(OH)2(��1��)
��2��2FeCl3+3Na2CO3+3H2O=2Fe(OH)3��+3CO2��+6NaCl(2��)
��3����Һ����ɫ��ɺ�ɫ(2��) Al3++3H2O![]() Al(OH)3+3H+(2��)
Al(OH)3+3H+(2��)
��4��500(2��)
��5��c(H+)��c(OH��)��c(CO32��)��c(HCO3��)��c(Na+)(2��)
��6��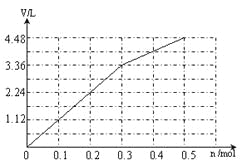 (2��)
(2��)
��������
��������֪��A��C��Ӧ�����ij�����Ӧ��������ͭ�����ᱵ��������A��C��CuSO4��Ba(OH)2�е�һ�֣�����֪��B��Eֻ�������࣬˫ˮ���������ͳ��������к���Fe3+��CO32���������б���Na2CO3������֪CΪǿ�����C��Ba(OH)2��A��CuSO4��DΪ���Σ����ڢ�֪��EΪNa2CO3������֪D����NO3��������DΪAl(NO3)3����Bֻ��ΪFeCl3��
��1��A��C�Ļ�ѧʽΪ��CuSO4��Ba(OH)2
��2����������Fe3+��CO32����˫ˮ�ⷴӦ����ѧ����ʽΪ2FeCl3+3Na2CO3+3H2O=2Fe(OH)3��+3CO2��+6NaCl��
��3��Al(NO3)3��Һ����Al3+ˮ�������Һ�����ԣ����Լ���ʯ����Һ����Һ���ɫ�����ӷ���ʽΪAl3++3H2O![]() Al(OH)3+3H+
Al(OH)3+3H+
��4������Cu��ϡ���ᷴӦ�����ӷ���ʽ3Cu+8H++2N3-=3Cu2++2NO��+4H2O����3Cu��8H+��38.4 g Cu�����ʵ���Ϊ0.6mol��������Ҫ��������ʵ�����0.8�������Ϊ500mL��
��5��������֪n(CO2)=0.5mol��n(Na2CO3)=1.5mol�����Զ��߷�Ӧ����Һ����1mol��NaHO3��1mol��Na2CO3��
Na2CO3ˮ��̶ȴ���NaHO3ˮ��̶ȣ�������Һ������Ũ����С�����˳��Ϊc(H+)��c(OH��)��c(CO32��)��c(HCO3��)��c(Na+)(2��)
��6����������Cl-�ŵ磬��Һ�й���0.3mol Cl-,����2 Cl-+2 e-= Cl2������ת�Ƶ���0.3molʱ���������״���µ������3.36L��Ȼ�������������ӷŵ磬4OH--4 e-=O2��+2H+��ÿת��0.1mol���Ӿ�����0.56L�����壬����ͼ�����£�