��Ŀ����
�������ӷ���ʽ��ȷ����
A���ö��Ե缫��ⱥ��ʳ��ˮ��2Cl��+2H2O Cl2��+H2��+2OH�� Cl2��+H2��+2OH�� |
B��������̼������Һ�� + CO32���� + CO32���� + H2CO3 + H2CO3 |
| C������������ʵ���Ũ�ȵ�NH4Fe(SO4)2��Ba(OH)2��ϣ�2Fe3++ 3SO42��+ 3Ba2++6OH����3BaSO4��+ 2Fe(OH)3�� |
| D����ǿ������Һ�д���������Fe(OH)3��Ӧ����Na2FeO4��3ClO��+2Fe(OH)3��2FeO42��+3Cl��+ H2O+4H+ |
AC
�������������B������̼�����ƣ�������ʵ�£�����D����ǿ������Һ�в�����������ģ�����
���㣺������Ҫ�������ӷ���ʽ����ȷ��д��

��ϰ��ϵ�д�
�����Ŀ
�������ӷ�Ӧ����ʽ��ȷ����
| A��������Ͷ������ˮ2 Na+2H2O��2 Na++2OH- +H2�� |
| B��������������Һ�м�����������SO3 2- +2 H+=SO2��+ H2O |
| C����Ũ������MnO2��Ӧ��ȡ��������MnO2+4H++2C1- = Mn2+ + 2H2O+Cl2�� |
| D������������Һ�м����ữ��˫��ˮFe2++2H++H2O2 = Fe3++2H2O |
���л�ѧ����ʽ�У����������ӷ���ʽH++OH-=H2O��ʾ����
| A��2NaOH+H2SO4=Na2SO4+2H2O | B��Ba(OH)2+2HCl=BaCl2+2H2O |
| C��KOH+HCl=KCl+H2O | D��Cu(OH)2+2HNO3=Cu(NO3)2+2H2O |
ij��Һ�п��ܴ���Fe3+��Fe2+��I-��HCO3-��Cl-��NO3-���������еļ��֡���������ʵ�飺
��ȡ������Һ�μ�KSCN��Һ����Һ��Ѫ��ɫ��
����ȡ����ԭ��Һ�μ����ᣬ��Һ���ػ�ɫ���
�ݴ˿����ƶϣ�����Һ�п϶����ܴ������ڵ�������
| A��I- | B��HCO3- | C��Fe2+ | D��NO3- |
�����£����и���������ָ����Һ��һ���ܴ����������
| A��0.1 mol��L-1��KI��Һ�� Na����Ag+��NO3-��SO42- |
| B��ʹ���ȱ��ɫ����Һ��NH4+��Cu2+��ClO����Cl�� |
| C��0.1 mol��L-1��KMnO4��Һ��Na����K����Cl����SO32- |
| D�������ӱ���ɫ����Һ��Na����Mg2+��SO42-��Cl�� |
�ں���Fe3+��Fe2+��Al3+��NH4+��ϡ��Һ�м���������Na2O2���壬��ַ�Ӧ���ټ��������ϡ���ᣬ��Ӧ��ȫ��������Ŀ����û�б仯����
| A��Fe3+ | B��Fe2+ | C��Al3+ | D��NH4+ |
�����£���c(H+)/c(OH?)��1��1012����Һ�У����и��������ܴ����������
| A��Fe2+��Mg2+��NO3?��SO42? | B��Fe3+��Na+��Cl?��SCN? |
| C��NH4+��Al3+��NO3?��Cl? | D��Ca2+��K+��Cl?��HCO3? |
�������ӷ���ʽ��ȷ���ǣ� ��
| A��������̼������Һ��Ӧ��2H+��CO32��== CO2����H2O |
| B������ʯ��ˮ�м��������̼��������Һ��Ca2+ + HCO + OH��= CaCO3�� + H2O |
C����������Һ��ͨ������������̼��2C6H5O����CO2��H2O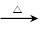 2C6H5OH��CO32�� 2C6H5OH��CO32�� |
D����ȩ��Һ��������������Һ����HCHO+4[Ag(NH3)2]++4OH��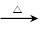 CO32��+2NH4++ 4Ag��+ 6NH3��+ 2H2O �� CO32��+2NH4++ 4Ag��+ 6NH3��+ 2H2O �� |
�±������ۺ������ǣ� ��
| ѡ�� | ��ѧ��Ӧ�������ӷ���ʽ | ���� |
| A | Fe3O4��ϡ���ᷴӦ��2Fe3O4+18H+ =6Fe3++H2��+8H2O | ��ȷ |
| B | ��̼��þ�м���ϡ���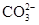 +2H+ =CO2��+H2O +2H+ =CO2��+H2O | ����̼��þ��Ӧд��������ʽ |
| C | ���������Һ�м�������������Һ�� Ba2++ 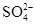 =" " BaSO4�� =" " BaSO4�� | ��ȷ |
| D | FeBr2��Һ������ʵ�����Cl2��Ӧ�� 2Fe2++2Br-+2Cl2=2Fe3++4Cl-+Br2 | ����Fe2+��Br-�Ļ�ѧ������֮��ӦΪ1��2 |