��Ŀ����
16�� ��15mL0.10mol•L-1NaOH ��Һ����μ��� 0.20mol•L-1 �������ᣨHCN��һԪ���ᣩ��Һ����Һ��pH�ͼ������������Һ�������ϵ������ͼ��ʾ���й�����Ũ�ȴ�С��ϵ��ȷ���ǣ�������
��15mL0.10mol•L-1NaOH ��Һ����μ��� 0.20mol•L-1 �������ᣨHCN��һԪ���ᣩ��Һ����Һ��pH�ͼ������������Һ�������ϵ������ͼ��ʾ���й�����Ũ�ȴ�С��ϵ��ȷ���ǣ�������| A�� | �� A��B ������һ�㣺c��Na+����c��CN-����c��OH-����c��H+�� | |
| B�� | �� B �㣺c��Na+���Tc��CN-����c�� OH-��=c��H+������ a=7.5 | |
| C�� | �� C �㣺c��CN-����c��Na+����c��OH-����c��H+�� | |
| D�� | �� D �㣺c��HCN��+c��CN-����2c��Na+�� |
���� A�������HCN���٣���c��OH-������c��CN-����
B��B�㣬��Һ�����ԣ���c��OH-��=c��H+�������������ǿ�������Σ�Ҫʹ��Һ�����ԣ�������ʵ���Ӧ�������ڼ
C��C�㣬pH��7����Һ�����ԣ�
D��D��������������������������ʵ�������KOH�����ʵ�����
��� �⣺A����A��B������һ�㣬��Ϊ��ʼʱc��OH-����c��CN-������Ҳ�п�����c��Na+����c��OH-����c��CN-����c��H+������A����
B��B�㣬��Һ�����ԣ���c��OH-��=c��H+����KCN��ǿ�������Σ�Ҫʹ��Һ�����ԣ�������ʵ���Ӧ�������ڼ����a��7.5����B����
C��C�㣬pH��7����Һ�����ԣ���c��H+����c��OH-�������ݵ���غ��c��CN-��+c��OH-��=c��H+��+c��Na+�������Ե�c��CN-����c��Na+������C����
D��D�����������������������������ʵ���Ϊ0.02L��0.2mol/L�T0.004mol��NaOH�����ʵ���Ϊ0.015L��0.1mol/L=0.0015mol����Ӧ��c��CN-��+c��HCN����2c��Na+������D��ȷ��
��ѡD��
���� ���⿼���������Һ�����жϼ��йؼ��㣬Ϊ��Ƶ���㣬���ؿ���ѧ����������������������Һ�е����ʼ��������ǽⱾ��ؼ�����ϵ���غ㼰�����غ�����������״�ѡ����D��

| A�� | PM2.5��ƿ���ο�����������Խϴ������к����� | |
| B�� | ú��������ʯ�͵ķ����������仯 | |
| C�� | �Ӻ�ˮ����ȡ���ʶ�����ͨ����ѧ��Ӧ����ʵ�� | |
| D�� | ���϶�����ʹ�õ�̼��ά��һ�����͵��л��߷��Ӳ��� |
| A�� | 3��4-�������� | B�� | 2��3-�������� | ||
| C�� | ���� | D�� | 2��2��3��3-�ļ����� |

�й������б����£�
| �Ҵ� | 1��2-�������� | ���� | |
| ״̬ | ��ɫҺ�� | ��ɫҺ�� | ��ɫҺ�� |
| �ܶ�/g•cm-3 | 0.79 | 2.2 | 0.71 |
| �е�/�� | 78.5 | 132 | 34.6 |
| �۵�/�� | -130 | 9 | -116 |
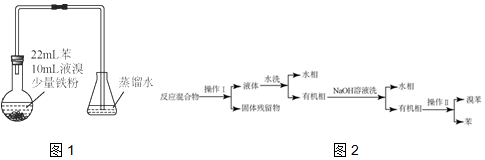
��1����װ��C��Ӧ����c��ѡ����ţ�����Ŀ�������շ�Ӧ�п������ɵ��������壮
��ˮ��Ũ���������������Һ �ܱ���̼��������Һ
��2���ж�d�����Ʊ��������鷴Ӧ�ѽ�����������������ɫ��ȫ��ȥ��
��3����������������δ��Ӧ��Br2������âڣ�����ȷѡ��ǰ����ţ�ϴ�ӳ�ȥ��
��ˮ������������Һ �۵⻯����Һ ���Ҵ�
��4����Ӧ������������ˮ��ȴ��װ��e��������ҪĿ���DZ�����Ĵ����ӷ��������ñ�ˮ���й�����ȴ��ԭ���ǣ�1��2-������������̵�ϵͣ�������ȴ��ʹ�����̶�ʹ��·������
��5����1��2-��������Ϊԭ�ϣ��Ʊ�������ϩ��Ϊ�����ԭ�������ʣ���ͬѧ������������̣�1��2-��������ͨ����ȥ��Ӧ�Ƶ���Ȳ����Ӧ�Ļ�ѧ����ʽΪ
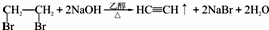 ����Ȳ�Ƶ�����ϩ�����������ϩ�Ƶ�����ϩ����Ӧ�Ļ�ѧ����ʽΪnCH2=CHCl$\stackrel{һ������}{��}$
����Ȳ�Ƶ�����ϩ�����������ϩ�Ƶ�����ϩ����Ӧ�Ļ�ѧ����ʽΪnCH2=CHCl$\stackrel{һ������}{��}$ ��
�� 
| A�� | a��b��Ϊͬ���칹�� | B�� | b��c��ͬϵ�� | ||
| C�� | a��b���ܷ����ӳɷ�Ӧ | D�� | ֻ��b��c�ܷ���ȡ����Ӧ |
 Һ��ͨ������CO2
Һ��ͨ������CO2 �����ӷ���ʽ��2ClO����CO2��H2O=2HClO��CO32-
�����ӷ���ʽ��2ClO����CO2��H2O=2HClO��CO32-
 ��
��