��Ŀ����
11��ijС��������ͼ1װ�ã��ñ�������FeBr3���������Ʊ��屽����Ӧ���ҽ��У���ƿ���д�������ɫ��������ƿ�е��ܿ��а������֣�����ˮ��ɻ�ɫ����Ӧֹͣ������������ͼ2�����Ʒ��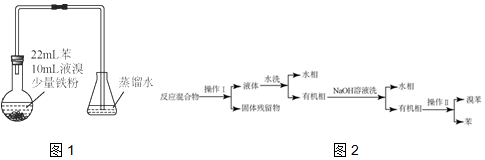
��֪���屽������ˮ�����л��ܼ����ܣ��塢�����屽�ķе�����Ϊ59�桢80�桢156�森
��1��д����ʵ�����Ʊ��屽�Ļ�ѧ����ʽ��
 ��
����2��������Ϊ���ˣ�������Ϊ����
��3����ˮϴ��������ˮ���еμ�KSCN��Һ����Һ���ɫ���Ʋ�ˮϴ����ҪĿ���dz�ȥFeBr3��
��4����ƿ������ˮ��Ƶ�ԭ�����ܽ��˴���ƿ�лӷ������壮
��5����֪�����巢����ȡ����Ӧ���ƲⷴӦ����ƿ��Һ�庬�е����ִ������ӣ������ʵ�鷽����֤����Ʋ⣮����ѡ�Լ���þ�������Ȼ�̼����ˮ����ˮ������ˮ��
| ��� | ʵ�鲽�� | Ԥ������ | ���� |
| 1 | |||
| 2 | ��ƿ��Һ�庬���� | ||
| 3 | ��ƿ��Һ�庬���� |
���� ��1����2����3����������FeBr3���������Ʊ��屽����Ӧ������к��б����屽���塢FeBr3�Լ�Fe�������̿�֪���������õ����������������ӦΪ���ˣ����������ΪFe�ۣ�Һ���к���FeBr3�ȣ�������ˮ��ˮϴ����з�Һ���룬�л����к��б����屽���壬��������������Һ��ȥBr2���پ�����Һ���룬ˮ������Ҫ����NaBr��NaBrO�ȣ��л����к��б����屽�����ڶ��߷е㲻ͬ���ɽ���������룻
��4�����ӷ����ӷ�����������ƿ��ˮ�У�ʹ����ˮ��ƣ�
��5�������巢������ȡ����Ӧ����Ӧ����HBr������ƿ�У���Һ�к��д�����Br-��H+���������ӷ�����ƿ�л��ܽ��������壬�����Ȼ�̼��Һ��ȡ���룬������ˮ�û����嵥�ʼ���Br-���ӣ�����Mg���ᷴӦ�����������H+���ӣ�
��� �⣺��1������Һ�巴Ӧ�����屽�Ļ�ѧ����ʽ�ǣ� ��
��
�ʴ�Ϊ�� ��
��
��2�������Ϸ�����֪������Ϊ���ˣ�������Ϊ���ʴ�Ϊ�����ˣ�����
��3����ˮϴ��������ˮ���еμ�KSCN��Һ����Һ���ɫ��˵��ˮ��Һ�к��������ӣ���ˮϴ����ҪĿ���dz�ȥ FeBr3���ʴ�Ϊ��FeBr3��
��4���������ӷ�����ƿ��ˮ�ܽ��˴���ƿ�лӷ����������ƣ��ʴ�Ϊ���ܽ��˴���ƿ�лӷ������壻
��5�������巢������ȡ����Ӧ����Ӧ����HBr������ƿ�У���Һ�к��д�����Br-��H+���������ӷ�����ƿ�л��ܽ��������壬�����Ȼ�̼��Һ��ȡ���룬�ֱ�ȡ�����ϲ���ɫ��Һ���Թ�A��B�У��Թ�A�е���������ˮ���û����嵥�ʣ���Һ����ɫ���ɫ��֤������Br-���ӣ���B�Թ��м���Mg�����д����������ɣ�֤������H+���ӣ�
�ʴ�Ϊ��
| ��� | ʵ�鲽�� | Ԥ������ | ���� |
| 1 | ����ƿ��Һ��ת���Һ©���������������Ȼ�̼�����Һ���ֱ�ȡ�����ϲ���ɫ��Һ���Թ�A��B�� | ||
| 2 | ���Թ�A�м���������ˮ | ��Һ����ɫ���ɫ | Br- |
| 3 | ���Թ�B�м���þ�� | �д����������� | H+ |
���� ���⿼���л�����Ʊ��Լ����ʵķ��롢�ᴿ��Ϊ�߿��������ͺ�Ƶ���㣬������ѧ���ķ�����ʵ�������Ŀ��飬ע�����ʵ���ԭ���Լ�ʵ�����̣�Ϊ������Ĺؼ����ѶȲ���

| A�� | �����ϩ������ʵ�����Br2������Ӧ | |
| B�� | 2-�ȶ�����NaOH�Ҵ���Һ���ȷ�����Ӧ | |
| C�� | ��ϩ��H2O�ڴ��������·�����Ӧ | |
| D�� | ���������������һ�ȴ���ķ�Ӧ |
 ��15mL0.10mol•L-1NaOH ��Һ����μ��� 0.20mol•L-1 �������ᣨHCN��һԪ���ᣩ��Һ����Һ��pH�ͼ������������Һ�������ϵ������ͼ��ʾ���й�����Ũ�ȴ�С��ϵ��ȷ���ǣ�������
��15mL0.10mol•L-1NaOH ��Һ����μ��� 0.20mol•L-1 �������ᣨHCN��һԪ���ᣩ��Һ����Һ��pH�ͼ������������Һ�������ϵ������ͼ��ʾ���й�����Ũ�ȴ�С��ϵ��ȷ���ǣ�������| A�� | �� A��B ������һ�㣺c��Na+����c��CN-����c��OH-����c��H+�� | |
| B�� | �� B �㣺c��Na+���Tc��CN-����c�� OH-��=c��H+������ a=7.5 | |
| C�� | �� C �㣺c��CN-����c��Na+����c��OH-����c��H+�� | |
| D�� | �� D �㣺c��HCN��+c��CN-����2c��Na+�� |
 �л���A��B�ķ���ʽΪC5H10O2�������������¾���ˮ�⣬ת����ϵ��ͼ�������й�˵���в���ȷ���ǣ�������
�л���A��B�ķ���ʽΪC5H10O2�������������¾���ˮ�⣬ת����ϵ��ͼ�������й�˵���в���ȷ���ǣ�������| A�� | X����Ϊ���ᣬҲ����Ϊ���� | B�� | C�����е�̼ԭ�������Ϊ3�� | ||
| C�� | C�����ܷ���������Ӧ | D�� | X��Y��Ϊͬ���칹�壮 |

 ��
�� ��
��