��Ŀ����
��֪ij��ҵ��ˮ�к��д���CuSO4��������Ag+��Hg2+�Լ��������࣬ͨ���������̿ɴӸ÷�ˮ�л�������ͭ���弰�������ʣ�
��1������1����Ҫ������
��2������2�������ij���Լ����ٽ��������룬���Լ��ǣ��ѧʽ��
��3������3���漰�IJ����ǣ�����Ũ����
��1������1����Ҫ������
����
����
�����õ��IJ����������ձ�����������©��
�ձ�����������©��
����2������2�������ij���Լ����ٽ��������룬���Լ��ǣ��ѧʽ��
Cu
Cu
����������ijɷ��ǣ��ѧʽ��Ag��Cu
Ag��Cu
����3������3���漰�IJ����ǣ�����Ũ����
��ȴ�ᾧ
��ȴ�ᾧ
�����ˡ���ɣ�
��������1�������Һ��ķ����ù��˵ķ������õ��ձ���©������������������
��2��Cu�����ʱ�Ag��Hg���ã�Ӧ���������Cu���ɷ����û���Ӧ����Ag��Hg���ɷ��������ע�ⲻ�������µ����ʣ�
��3������Һ�л�ȡ�ᾧˮ����辭������Ũ������ȴ�ᾧ�����ˡ���ɵȲ�����
��2��Cu�����ʱ�Ag��Hg���ã�Ӧ���������Cu���ɷ����û���Ӧ����Ag��Hg���ɷ��������ע�ⲻ�������µ����ʣ�
��3������Һ�л�ȡ�ᾧˮ����辭������Ũ������ȴ�ᾧ�����ˡ���ɵȲ�����
����⣺��1������1Ϊ�����Һ��ķ��룬Ϊ���˲������õ��ձ���©������������������ȱ�ٵ����ձ���©�������������ʴ�Ϊ���ձ���©������������
��2��Cu�����ʱ�Ag��Hg���ã�Ӧ���������Cu���ɷ����û���Ӧ����Ag��Hg���ɷ��������ע�ⲻ�������µ����ʣ�������������Լ�ӦΪCu��Hg�ڳ�����ΪҺ�壬��������ΪAg��Cu���ʴ�Ϊ��Cu�� Ag��Cu��
��3������Һ�л�ȡ�ᾧˮ����辭������Ũ������ȴ�ᾧ�����ˡ���ɵȲ������ʴ�Ϊ����ȴ�ᾧ��
��2��Cu�����ʱ�Ag��Hg���ã�Ӧ���������Cu���ɷ����û���Ӧ����Ag��Hg���ɷ��������ע�ⲻ�������µ����ʣ�������������Լ�ӦΪCu��Hg�ڳ�����ΪҺ�壬��������ΪAg��Cu���ʴ�Ϊ��Cu�� Ag��Cu��
��3������Һ�л�ȡ�ᾧˮ����辭������Ũ������ȴ�ᾧ�����ˡ���ɵȲ������ʴ�Ϊ����ȴ�ᾧ��
���������⿼�����ʵķ���֪ʶ�����Ը�����ѧ֪ʶ���лش���Ŀ�ѶȲ���

��ϰ��ϵ�д�
 Ʒѧ˫�ž�ϵ�д�
Ʒѧ˫�ž�ϵ�д� Сѧ��ĩ���100��ϵ�д�
Сѧ��ĩ���100��ϵ�д� ��ĩ��ϰ���ϵ�д�
��ĩ��ϰ���ϵ�д� ����ѧ�䵥Ԫ������ĩר����100��ϵ�д�
����ѧ�䵥Ԫ������ĩר����100��ϵ�д�
�����Ŀ

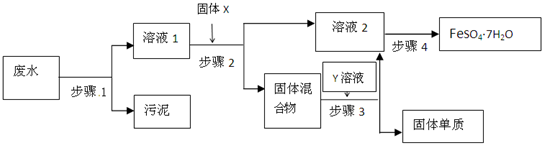

 ��֪ij��ҵ��ˮ�к���N
��֪ij��ҵ��ˮ�к���N