ΧβΡΩΡΎ»ί
ΓΨΧβΡΩΓΩΫπ τΡχ”–ΙψΖΚΒΡ”ΟΆΨΘ§¥÷Ρχ÷–Κ§”–…ΌΝΩFeΓΔZnΓΔCuΓΔPtΒ»‘”÷ Θ§“‘ΝρΥαΡχ »ή“ΚΈΣΒγΫβ“ΚΫχ––¥÷Ρχ Χα¥ΩΘ§œ¬Ν–ΥΒΖ®’ΐ»ΖΒΡ «Θ®“―÷ΣΘΚ―θΜ·–‘ΒΡ«Ω»θΥ≥–ρΈΣFe2+ΘΦNi2+ΘΦCu2+Θ©
A. ―τΦΪ Ήœ»ΖΔ…ζΒΡΒγΦΪΖ¥”Π «Ni2++2e-=Ni
B. ΒγΫβΙΐ≥Χ÷–Θ§―τΦΪ÷ ΝΩΒΡΦθ…Ό”κ“θΦΪ÷ ΝΩΒΡ‘ωΦ”œύΒ»
C. ΒγΫβΚσΘ§»ή“Κ÷–¥φ‘ΎΫπ τ―τάκΉ”÷Μ”–Fe2+ΚΆZn2+
D. ΒγΫβΚσΘ§CuΚΆPt≥ΝΜΐ‘ΎΒγΫβ≤έΒΉ≤Ω–Έ≥…―τΦΪΡύ
ΓΨ¥πΑΗΓΩD
ΓΨΫβΈωΓΩΧα¥Ω¥÷Ρχ ±Θ§¥÷ΡχΈΣ―τΦΪΘ§¥ΩΡχΈΣ“θΦΪΓΘAœνΘ§―θΜ·–‘ΒΡ«Ω»θΥ≥–ρΈΣFe2+ΘΦNi2+ΘΦCu2+Θ§ΜΙ‘≠–‘ΒΡ«Ω»θΥ≥–ρΈΣFe![]() Ni
Ni![]() CuΘ§ΫαΚœΫπ τΜνΕ·–‘Υ≥–ρ±μΘ§―τΦΪ Ήœ»ΖΔ…ζΒΡΒγΦΪΖ¥”Π «ΘΚZn-2e-=Zn2+Θ§¥μΈσΘΜBœνΘ§ΒγΫβΙΐ≥Χ÷–Θ§―τΦΪ“ά¥ΈΖΔ…ζΒΡΒγΦΪΖ¥”ΠΈΣΘΚZn-2e-=Zn2+ΓΔFe-2e-=Fe2+ΓΔNi-2e-=Ni2+Θ§“θΦΪΒΡΒγΦΪΖ¥”ΠΈΣNi2++2e-=NiΘ§”…”Ύ¥÷Ρχ÷–ΗςΈο÷ ΒΡΚ§ΝΩΈ¥÷ΣΘ§―τΦΪ÷ ΝΩΒΡΦθ…Ό”κ“θΦΪ÷ ΝΩΒΡ‘ωΦ”≤Μ“ΜΕ®œύΒ»Θ§¥μΈσΘΜCœνΘ§ΒγΫβΚσ»ή“Κ÷–¥φ‘ΎΒΡΫπ τ―τάκΉ”ΈΣZn2+ΓΔFe2+ΓΔNi2+Θ§¥μΈσΘΜDœνΘ§CuΓΔPt≤ΜΦΑNiΜνΤΟΘ§‘Ύ―τΦΪ≤ΜΖ≈ΒγΘ§≥ΝΜΐ‘ΎΒγΫβ≤έΒΉ≤Ω–Έ≥…―τΦΪΡύΘ§’ΐ»ΖΘΜ¥πΑΗ―ΓDΓΘ
CuΘ§ΫαΚœΫπ τΜνΕ·–‘Υ≥–ρ±μΘ§―τΦΪ Ήœ»ΖΔ…ζΒΡΒγΦΪΖ¥”Π «ΘΚZn-2e-=Zn2+Θ§¥μΈσΘΜBœνΘ§ΒγΫβΙΐ≥Χ÷–Θ§―τΦΪ“ά¥ΈΖΔ…ζΒΡΒγΦΪΖ¥”ΠΈΣΘΚZn-2e-=Zn2+ΓΔFe-2e-=Fe2+ΓΔNi-2e-=Ni2+Θ§“θΦΪΒΡΒγΦΪΖ¥”ΠΈΣNi2++2e-=NiΘ§”…”Ύ¥÷Ρχ÷–ΗςΈο÷ ΒΡΚ§ΝΩΈ¥÷ΣΘ§―τΦΪ÷ ΝΩΒΡΦθ…Ό”κ“θΦΪ÷ ΝΩΒΡ‘ωΦ”≤Μ“ΜΕ®œύΒ»Θ§¥μΈσΘΜCœνΘ§ΒγΫβΚσ»ή“Κ÷–¥φ‘ΎΒΡΫπ τ―τάκΉ”ΈΣZn2+ΓΔFe2+ΓΔNi2+Θ§¥μΈσΘΜDœνΘ§CuΓΔPt≤ΜΦΑNiΜνΤΟΘ§‘Ύ―τΦΪ≤ΜΖ≈ΒγΘ§≥ΝΜΐ‘ΎΒγΫβ≤έΒΉ≤Ω–Έ≥…―τΦΪΡύΘ§’ΐ»ΖΘΜ¥πΑΗ―ΓDΓΘ

ΓΨΧβΡΩΓΩH2O2ΉςΈΣ―θΜ·ΦΝ‘ΎΖ¥”Π ±≤Μ≤ζ…ζΈέ»ΨΈο±Μ≥ΤΈΣ¬Χ…Ϊ―θΜ·ΦΝΘ§“ρΕχ ήΒΫ»ΥΟ«‘Ϋά¥‘ΫΕύΒΡΙΊΉΔΓΘΈΣ±»ΫœFe3ΘΪΚΆCu2ΘΪΕ‘H2O2Ζ÷ΫβΒΡ¥ΏΜ·–ßΙϊΘ§Ρ≥Μ·―ß―–ΨΩ–ΓΉιΒΡΆ§―ßΖ÷±π…ηΦΤΝΥ»γΆΦΦΉΓΔ““Υυ ΨΒΡ Β―ιΓΘ«κΜΊ¥πœ¬Ν–Έ ΧβΘΚ
Θ®1Θ©Ε®–‘Ζ÷ΈωΘΚΆΦΦΉΩ…Ά®ΙΐΙέ≤λ________________Ε®–‘±»ΫœΒΟ≥ωΫα¬έΓΘ”–Ά§―ßΧα≥ωΫΪFeCl3»ή“ΚΗΡΈΣFe2(SO4)3»ή“ΚΗϋΚœάμΘ§Τδάμ”… «_______________________ΓΘ

Θ®2Θ©Ε®ΝΩΖ÷ΈωΘΚ»γΆΦ““Υυ ΨΘ§ Β―ι ±Ψυ“‘…ζ≥…40 mLΤχΧεΈΣΉΦΘ§ΤδΥϊΩ…Ρή”Αœλ Β―ιΒΡ“ρΥΊΨυ“―Κω¬‘ΓΘΆΦ÷–“«ΤςAΒΡΟϊ≥ΤΈΣ_______Θ§Φλ≤ιΗΟΉΑ÷ΟΤχΟή–‘ΒΡΖΫΖ® «ΘΚΙΊ±’AΒΡΜν»ϊΘ§ΫΪΉΔ…δΤςΜν»ϊœρΆβά≠≥ω“ΜΕΈΚσΥ… ÷Θ§Ιΐ“ΜΕΈ ±ΦδΚσΩ¥__________Θ§ Β―ι÷––η“Σ≤βΝΩΒΡ ΐΨί «__________ΓΘ
Θ®3Θ©Φ”»κMnO2ΖέΡ©”ΎH2O2»ή“Κ÷–Θ§‘Ύ±ξΉΦΉ¥Ωωœ¬Ζ≈≥ωΤχΧεΒΡΧεΜΐΚΆ ±ΦδΒΡΙΊœΒ»γΆΦΥυ ΨΓΘ

”…DΒΫAΙΐ≥Χ÷–Θ§ΥφΉ≈Ζ¥”ΠΒΡΫχ––Ζ¥”ΠΥΌ¬ ÷πΫΞ_________ΓΘΘ®Χν ΓΑΦ”ΩλΓ±ΜρΓΑΦθ¬ΐΓ±Θ©,Τδ±δΜ·ΒΡ‘≠“ρ «_____________ΓΘΘ®―ΓΧνΉ÷ΡΗ±ύΚ≈Θ©
A.ΗΡ±δΝΥΖ¥”ΠΒΡΜνΜ·Ρή B.ΗΡ±δΜνΜ·Ζ÷Ή”ΑΌΖ÷±»
C.ΗΡ±δΝΥΖ¥”ΠΆΨΨΕ D.ΗΡ±δΒΞΈΜΧεΜΐΡΎΒΡΖ÷Ή”Ήή ΐ
Θ®4Θ©Νμ“Μ–ΓΉιΆ§―ßΈΣΝΥ―–ΨΩ≈®Ε»Ε‘Ζ¥”ΠΥΌ¬ ΒΡ”ΑœλΘ§…ηΦΤΝΥ»γœ¬ Β―ι…ηΦΤΖΫΑΗΘ§«κΑο÷ζΆξ≥…(Υυ”–Ω’Ψυ–ηΧν¬ζΘ©ΓΘ
Β―ι±ύΚ≈ | T/K | ¥ΏΜ·ΦΝ | ≈®Ε» |
Β―ι1 | 298 | 3ΒΈFeCl3»ή“Κ | 10ml 2%H2O2 |
Β―ι2 | 298 |
ΔΌ ‘Ύ Β―ι2÷–“ά¥ΈΧν_______________ΓΔ________________ΓΘ
ΔΎ Ω…ΒΟΒΫΒΡΫα¬έ «≈®Ε»‘Ϋ¥σΘ§H2O2 Ζ÷ΫβΥΌ¬ __________ΓΘ
ΓΨΧβΡΩΓΩœ¬ΆΦ «―άΗύ÷–Ρ≥–©÷ς“Σ≥…Ζ÷Φλ―ιΝς≥ΧΆΦΘ§«κΜΊ¥π“‘œ¬Έ ΧβΘΚ
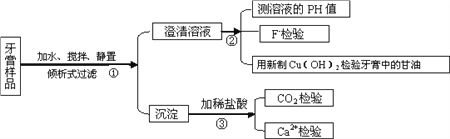
Θ®1Θ©―άΗύ÷–ΒΡΡΠ≤ΝΦΝ‘Φ’Φ―άΗύ≥…Ζ÷ΒΡ50%Θ§÷ς“Σ”Ο”Ύ‘ω«Ω―άΗύΒΡΡΠ≤ΝΉς”ΟΚΆ»ΞΈέ–ßΙϊΘ§≤ΜΆ§÷÷άύΒΡ―άΗύΚ§”–ΒΡΡΠ≤ΝΦΝ”–Υυ≤ΜΆ§Θ§‘Ύ…œ ωΝς≥ΧΆΦΒΡΒΎΔέ≤ΫΦ”»κ―ΈΥαΚσΜα≤ζ…ζ≤ΜΆ§œ÷œσΘ§«κΡψΧνΩ’ΘΚ
ΡΠ≤ΝΦΝ | Β―ι÷–≥ωœ÷ΒΡœ÷œσ | Ϋβ Ά |
SiO2 | ________________ | _____________ |
CaCO3 | ________________ | ______________ |
Ca3(PO4)2 | »ήΫβΈόΤχΧεΘ§Φ”NaOH÷ΝΙΐΝΩΚσ”÷≤ζ…ζ≥ΝΒμ | ______________ |
Al(OH)3 | »ήΫβΈόΤχΧεΘ§Φ”NaOH÷ΝΙΐΝΩœ»≤ζ…ζ≥ΝΒμΚσ»ήΫβ | _______________ |
Θ®2Θ©»’≥Θ…ζΜν÷– Ι”ΟΒΡ―άΗύάοΟφΕΦΚ§”–“ΜΕ®ΝΩΒΡΡΠ≤ΝΦΝΘ§ ‘ΜΊ¥πœ¬Ν–œύΙΊΈ ΧβΘΚ
AΘ°ΗυΨίΡψΒΡΆΤ≤βΘ§ΡΠ≤ΝΦΝ”ΠΨΏ±Ηœ¬Ν––‘÷ ÷–ΒΡΡΡΦΗΧθΘΩ________________
ΔΌ“Ή»ή”ΎΥ° ΔΎΡ―»ή”ΎΥ° ΔέΦα”≤ Δή»α»μ ΔίΩ≈ΝΘΫœ¥σ ΔόΩ≈ΝΘΫœ–Γ
BΘ°ΨίΒς≤ιΘ§ΝΫΟφ’κΕυΆ·―άΗύΓΔ’δ÷ιΆθΖά≥τ―άΗύΚΆ÷–ΜΣΆΗΟς―άΗύ÷–Υυ Ι”ΟΒΡΡΠ≤ΝΦΝ“ά¥Έ ««β―θΜ·¬ΝΓΔΧΦΥαΗΤΚΆΕΰ―θΜ·ΙηΘ§ΥϋΟ«Υυ τΒΡΈο÷ άύ±π“ά¥Έ «________________ΓΔ____________ΓΔ___________ΓΘ
CΘ°Ής―άΗύΡΠ≤ΝΦΝΒΡΖέΡ©Ή¥ΧΦΥαΗΤΩ…“‘”Ο ·Μ“ ·ά¥÷Τ±ΗΓΘ“‘œ¬ «“Μ÷÷÷Τ±ΗΧΦΥαΗΤΒΡ…ζ≤ζΖΫΑΗΘ§ΤδΝς≥ΧΆΦΈΣΘ§«κΡψ–¥≥ω…œ ωΖΫΑΗ÷–”–ΙΊΖ¥”ΠΒΡΜ·―ßΖΫ≥Χ ΫΘΚ
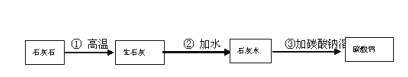
ΔΌ_______________ ΔΎ________________ Δέ___________________
Θ®3Θ©–¬÷ΤCuΘ®OHΘ©2ΒΡ≈δ÷ΤΖΫΖ® «__________________________ΓΘ
―άΗύ÷–Κ§”–“ΜΕ®ΝΩΒΡΗ ”ΆΘ§Τδ÷ς“ΣΙΠΡή «Ής±Θ ΣΦΝΘ§‘ΎΝς≥ΧΆΦΒΡΒΎΔΎ≤ΫΒΟΒΫΒΡ≥Έ«ε»ή“Κ÷–Φ”»κ–¬÷ΤCuΘ®OHΘ©2Θ§≤ζ…ζΒΡœ÷œσ «_____Θ§Μ·―ßΖΫ≥Χ ΫΈΣ_________
Θ®4Θ© Β―ι≤βΕ®≥Έ«ε»ή“ΚΒΡpH>7Θ§Ω…Ρή‘≠“ρ «___________ΓΘ
Θ®5Θ©―ά≥ί±μΟφ”…“Μ≤ψ”≤ΒΡΓΔΉι≥…ΈΣCa5(PO4)3OHΒΡΈο÷ ±ΘΜΛΉ≈Θ§Υϋ‘ΎΆΌ“Κ÷–¥φ‘Ύœ¬Ν–ΤΫΚβΘΚCa5(PO4)3OH(ΙΧ) ![]() 5Ca2++3PO43-+OH-
5Ca2++3PO43-+OH-
Ϋχ ≥ΚσΘ§œΗΨζΚΆΟΗΉς”Ο”Ύ ≥ΈοΘ§≤ζ…ζ”–ΜζΥαΘ§’β ±―ά≥ίΨΆΜα ήΒΫΗ· ¥Θ§Τδ‘≠“ρ «_____________________
“―÷ΣCa5(PO4)3FΘ®ΙΧΘ©ΒΡ»ήΫβΕ»±»…œΟφΒΡΩσΜ·≤ζΈοΗϋ–ΓΘ§÷ ΒΊΗϋΦαΙΧΓΘ”ΟάκΉ”ΖΫ≥Χ Ϋ±μ ΨΘ§Β±―άΗύ÷–≈δ”–ΖζΜ·ΈοΧμΦ”ΦΝΚσΡήΖά÷Ι»Θ≥ίΒΡ‘≠“ρΘΚ_____________Θ§ΗυΨί“‘…œ‘≠άμΘ§«κΡψΧα≥ω“Μ÷÷ΤδΥϋ¥ΌΫχΩσΜ·ΒΡΖΫΖ®_________________________
ΓΨΧβΡΩΓΩ“ΜΕ®Έ¬Ε»œ¬Θ§‘Ύ»ΐΗω»ίΜΐΨυΈΣ2.0 LΒΡΚψ»ίΟή±’»ίΤς÷–ΖΔ…ζΖ¥”ΠΘΚ
2NO(g)ΘΪ2CO(g)![]() N2(g)ΘΪ2CO2(g)
N2(g)ΘΪ2CO2(g)
Ης»ίΤς÷–Τπ ΦΈο÷ ΒΡΝΩ”κΖ¥”ΠΈ¬Ε»»γœ¬±μΥυ ΨΘ§Ζ¥”ΠΙΐ≥Χ÷–ΦΉΓΔ±ϊ»ίΤς÷–CO2ΒΡΈο÷ ΒΡΝΩΥφ ±Φδ±δΜ·ΙΊœΒ»γΆΦΥυ ΨΘΚ
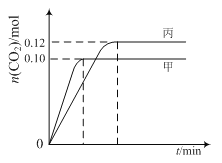
»ίΤς | Έ¬Ε»/Γφ | Τπ ΦΈο÷ ΒΡΝΩ/mol | |
NO (g) | CO (g) | ||
ΦΉ | T1 | 0.20 | 0.20 |
““ | T1 | 0.30 | 0.30 |
±ϊ | T2 | 0.20 | 0.20 |
œ¬Ν–ΥΒΖ®’ΐ»ΖΒΡ «
A. ΗΟΖ¥”ΠΒΡ’ΐΖ¥”ΠΈΣΈϋ»»Ζ¥”Π
B. ¥οΒΫΤΫΚβ ±Θ§““÷–CO2ΒΡΧεΜΐΖ÷ ΐ±»ΦΉ÷–ΒΡ–Γ
C. T1Γφ ±Θ§»τΤπ Φ ±œρΦΉ÷–≥δ»κ0.40 mol NOΓΔ0.40mol COΓΔ0.40mol N2ΚΆ0.40mol CO2Θ§‘ρΖ¥”Π¥οΒΫ–¬ΤΫΚβ«Αv(’ΐ)ΘΨv(Ρφ)
D. T2Γφ ±Θ§»τΤπ Φ ±œρ±ϊ÷–≥δ»κ0.06mol N2ΚΆ0.12 mol CO2Θ§‘ρ¥οΤΫΚβ ±N2ΒΡΉΣΜ·¬ ¥σ”Ύ40%