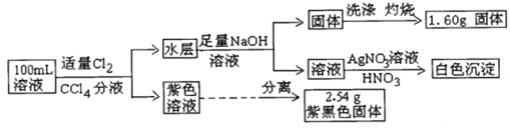
��Ŀ����
����Ŀ�������ᣨH3PO2����һ�־�ϸ������Ʒ����һԪ��ǿ�ᣬ���н�ǿ��ԭ�ԡ��ش��������⣺
��1��H3PO2�����������������Ʒ�Ӧ���ɵ�NaH2PO2���ɽ���Һ�е�Ag+��ԭΪAg���Ӷ������ڻ�ѧ������
����H3PO2�У���Ԫ�صĻ��ϼ�Ϊ________�����Ի����У����ã�H3PO2�����л�ѧ������Ӧ�У��������뻹ԭ�������ʵ���֮��Ϊ4��1������������Ϊ��________���ѧʽ����
��NaH2PO2��________������������������ʽ������������Һ������Ũ���ɴ�С��˳��ӦΪ________
��2�������ᣨH3PO2������ͨ�����ķ����Ʊ�������ԭ������ͼ��ʾ����Ĥ����Ĥ�ֱ�ֻ���������ӡ�������ͨ������

��д�������ĵ缫��Ӧʽ________
�ڷ�����Ʒ�ҿɵõ�H3PO2��ԭ��________�����һ�������ַ�����
���𰸡�+1 H3PO4 ���� c��Na+����c��H2PO2-����c��OH-����c��H+�� 2H2O-4e- = O2��+4H+ �����ҵ�H+������Ĥ��ɢ����Ʒ�ң�ԭ���ҵ�H2PO2-������Ĥ��ɢ����Ʒ�ң����߷�Ӧ����H3PO2
��������
��1����H3PO2����Ԫ��Ϊ+1�ۣ���Ԫ��Ϊ-2�ۣ����ݻ������л��ϼ۴�����Ϊ0�ɵã���Ԫ�صĻ��ϼ�Ϊ+1�ۣ�����H3PO2���л�ѧ������Ӧʱ��Ag+Ϊ��������H3PO2Ϊ��ԭ�������ߵ����ʵ���֮��Ϊ4:1������ת�Ƶ����غ�ɵã���Ӧ����Ԫ�صĻ��ϼ�Ϊ+5�ۣ������������ΪH3PO4���ʴ�Ϊ��+1��H3PO4
����ΪH3PO2��һԪ��ǿ�ᣬֻ�ܵ����һ��H+�����NaH2PO2Ϊ���Σ�ˮ��Һ��H2PO2-ֻ����ˮ�ⷴӦ�����������룬�����Һ�Լ��ԣ�����Һ������Ũ�ȣ�c(Na��)>c(H2PO2��)>c(OH��)>c(H��)���ʴ�Ϊ�����Σ�c(Na��)>c(H2PO2��)>c(OH��)>c(H��)��
��2����H2O���������OH-����������ʧ���ӵ�������Ӧ������O2���ʴ�Ϊ��![]() ��
��
��������Ӧ��H���� H��ͨ�������ӽ���Ĥ�����Ʒ���У������ĵ缫��ӦʽΪ![]() ��ԭ���ҵ�Na�����������ҡ�H2PO2�������Ʒ�ң��γ�H3PO2���ʴ�Ϊ�������ҵ�H+������Ĥ��ɢ����Ʒ�ң�ԭ���ҵ�H2PO2-������Ĥ��ɢ����Ʒ�ң����߷�Ӧ����H3PO2��
��ԭ���ҵ�Na�����������ҡ�H2PO2�������Ʒ�ң��γ�H3PO2���ʴ�Ϊ�������ҵ�H+������Ĥ��ɢ����Ʒ�ң�ԭ���ҵ�H2PO2-������Ĥ��ɢ����Ʒ�ң����߷�Ӧ����H3PO2��

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�