��Ŀ����
ΪӦ��ʯ��Σ����2006��1��1�գ���ʡ���ձ����С�����ȫ���ƹ�ʹ���Ҵ�������Ϊ������ȼ�ϣ����������в���һ���������Ҵ����Դ���һ�������͡������й�˵����ȷ����
| A���Ҵ�������һ�������Դ��ȼ�ղ��������Ⱦ |
| B���Ҵ����������Ԫ����ͬ����ѧ�ɷ����� |
| C���Ҵ�����ȼ��ʱ�����������ڵ����������� |
| D���Ҵ���ͨ������ת���Ƶã���һ�ֿ�������ȼ�� |
D
��

��ϰ��ϵ�д�
 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
�����Ŀ
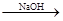 Mg(OH)2
Mg(OH)2 Mg
Mg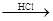 MgCl2��Һ
MgCl2��Һ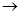 MgCl2����
MgCl2����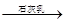 Mg(OH)2
Mg(OH)2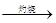 MgO
MgO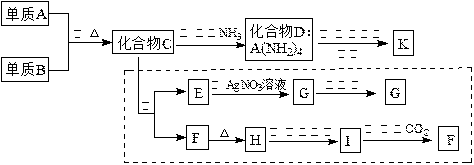
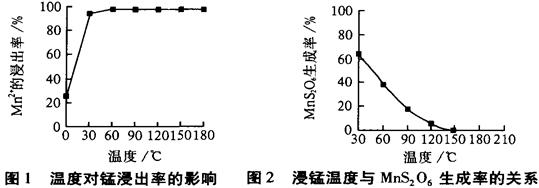
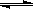 MnS2O6�ġ�H 0�����������Ϊ����MnS2O6�����ɣ������̡��������¶��� ��
MnS2O6�ġ�H 0�����������Ϊ����MnS2O6�����ɣ������̡��������¶��� ��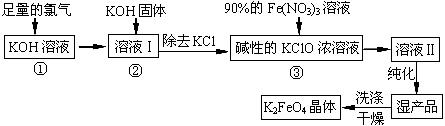
 ���ٶ���ʻ��40km��Ϊ����Ч��չ��������Դ�����ȱ����Ƶ����۵����������мȿ����־��õ����ⷽ���� �������������� ����д��ţ�
���ٶ���ʻ��40km��Ϊ����Ч��չ��������Դ�����ȱ����Ƶ����۵����������мȿ����־��õ����ⷽ���� �������������� ����д��ţ� ��4��������������ȼ������ˮ���Ȼ�ѧ����ʽ
��4��������������ȼ������ˮ���Ȼ�ѧ����ʽ
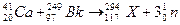 ���ñ仯�����ڻ�ѧ�仯
���ñ仯�����ڻ�ѧ�仯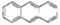 �����÷���������ԭ�ӿ��Դ���ͬһƽ��
�����÷���������ԭ�ӿ��Դ���ͬһƽ��