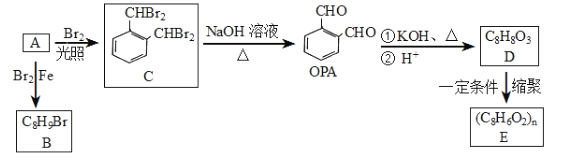
��Ŀ����
����Ŀ������β����Ⱦ�����������������Ҫԭ��֮һ����������������������������⣬����Ҫ�����з�������β����ת���������ü����д����������ѡ������ǹؼ���ijϡ������ת������β��ʾ��ͼ��ͼ��

��1�������й�˵����ȷ����__________
a.C5H8��CH3CH=CH2һ����������̼ԭ�Ӷ����õ���sp3�ӻ�
b.H2O��CO2��N2���ǷǼ��Է���
c��ÿ��CO2�����У�����2��������2������
d��CO��һ�ֵȵ�����ΪNO�������ĵ���ʽΪ[��NO��]��
��2��CO��Fe�������ʻ���[Fe(CO)5]����֪������Ϊ0�ۣ���ԭ���ڻ�̬��������Ų�ʽΪ___________��[Fe(CO)5]����λԭ����________��������________��
��3��C��N��O����Ԫ�صĵ�һ�������ɴ�С��˳����________��Al2O3�����۵�����ͻ���ϣ�AlCl3�������������۵�ͣ���ҵ�ϵ�ұ��ȡ����ǰ�߶����ú��ߵ�ԭ����________��
��4�����ѿ�����������Ҳ����Ϊ����β��ת���Ĵ�����һ�ָ��������ᄃ���ṹ����ͼ������ÿ��Sr2+��_____��O2-���ڣ���Sr2+��O2-���ڵĺ˼��Ϊapm������٤������ΪNA����������ᄃ���ܶȵļ������ʽΪ_________��

���𰸡�b�� d1s22s22p63s23p63d64s2��[Ar]3d64s2C������C�ĵ縺��С��O��Cԭ�Ӻ˶Թµ��ӶԵ���������Cԭ���ṩ�µ��Ӷ���λ����ǿ��Oԭ�ӡ�N>O>CAlCl3�������������۵�ͣ��Ƿ��Ӿ��壬����ʱ�����磬�����12![]() g/cm3
g/cm3
��������(1)a��̼̼˫���ϵ�Cԭ�Ӳ���sp2�ӻ�����a����b��H2O���Լ��Լ���ϵļ��Է��ӣ�CO2�к��м��Լ���Ϊֱ���η��ӣ��ṹ�Գƣ�������������������ص���Ϊ�Ǽ��Է��ӣ�N2���ԷǼ��Լ����ɵĵ��ʣ�Ϊ�Ǽ��Է��ӣ���b����c��CO2���ӵĽṹʽΪO=C=O��ÿ��CO2�����к���2���м���2���Ҽ�����c��ȷ��d��CO��һ�ֵȵ�����ΪNO+��CO�ĽṹΪC��O����NO+�ĵ���ʽΪ![]() ����d��ȷ����Ϊcd��
����d��ȷ����Ϊcd��
(2)CO��Fe�������ʻ���[Fe(CO)5]����֪������Ϊ0�ۣ���ԭ�ӵĺ˵����Ϊ26�����ڻ�̬��������Ų�ʽΪ1s22s22p63s23p63d64s2��[Ar]3d64s2�� ������C�ĵ縺��С��O��Cԭ�Ӻ˶Թµ��ӶԵ���������Cԭ���ṩ�µ��Ӷ���λ����ǿ��Oԭ�ӣ���[Fe(CO)5]����λԭ����C��
(3)ͬ����Ԫ�ص�һ�����ܳ��������ƣ���N��p�������3�����ӣ�Ϊ�����������ȶ��ṹ����C��N��O����Ԫ�صĵ�һ�������ɴ�С��˳����N>O>C��Al2O3�����۵�������Ӿ��壬��AlCl3�������������۵���Ƿ��Ӿ��壬���Ӿ����ۻ�ʱ�����粻�ܵ�⣬�ʵ�ұ��ȡ����Al2O3������AlCl3��
(4)Sr2+�ھ��������ģ���֮���ڵ�O2-�ھ��������ϣ�12���⣬ÿ��Sr2+���ڵ�O2-��12����Sr2+��O2-���ڵĺ˼��Ϊa��10-10cm������Sr2+Ϊͬһƽ���ϵ�4��O2-�γɵ������ε����ģ��������εı߳��������߳�Ϊ![]() a��10-10cm�����������Ϊ
a��10-10cm�����������Ϊ![]() cm3��ÿ�������к�����������12��
cm3��ÿ�������к�����������12��![]() =3��Sr2+ԭ��Ϊ1����Ti4+�ĸ���Ϊ8��
=3��Sr2+ԭ��Ϊ1����Ti4+�ĸ���Ϊ8��![]() =1�������Ļ�ѧʽΪTiSrO3��Ħ������Ϊ184g/mol�������ܶ�Ϊ
=1�������Ļ�ѧʽΪTiSrO3��Ħ������Ϊ184g/mol�������ܶ�Ϊ![]() g/cm3��
g/cm3��

 ����ѧ��Ӯ�����ϵ�д�
����ѧ��Ӯ�����ϵ�д� ѧ���쳵�����ּ��������ҵ�½����������ϵ�д�
ѧ���쳵�����ּ��������ҵ�½����������ϵ�д� �����ѧСѧ�꼶�νӵ������㽭��ѧ������ϵ�д�
�����ѧСѧ�꼶�νӵ������㽭��ѧ������ϵ�д�