��Ŀ����
(8��)A��B��C��D��E����Ԫ�����ڱ���ǰ20��Ԫ�أ�ԭ��������������B��C��Dͬ���ڣ���A��Dͬ���壬E������Ԫ�ؼȲ���ͬ����Ҳ����ͬ���壬B��C��D������������ˮ����������Ͼ��ܷ�����Ӧ�����κ�ˮ��
����������Ϣ���ش��������⣺
(1)A��D�⻯���У��е�ϵ͵��� (ѡ�A����D��)��A��B�������У��뾶��С���� (�����ӷ���)��
(2)Ԫ��C��Ԫ�����ڱ��е�λ���� ��
(3)A��E��������ӻ�����侧��(�������ھ����о��д����Ե���С�ظ���Ԫ)�ṹ����ͼ��ʾ��������(�á���ʾ)λ�ڸ�������Ķ�������ģ�������(�á�������ʾ)��λ��С���������ġ��û�����ĵ���ʽ�� ��
����������Ϣ���ش��������⣺
(1)A��D�⻯���У��е�ϵ͵��� (ѡ�A����D��)��A��B�������У��뾶��С���� (�����ӷ���)��
(2)Ԫ��C��Ԫ�����ڱ��е�λ���� ��
(3)A��E��������ӻ�����侧��(�������ھ����о��д����Ե���С�ظ���Ԫ)�ṹ����ͼ��ʾ��������(�á���ʾ)λ�ڸ�������Ķ�������ģ�������(�á�������ʾ)��λ��С���������ġ��û�����ĵ���ʽ�� ��

(1)D Na+ (2)�������ڡ���A�� (3) 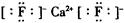 (ÿ��2�֣���8��)
(ÿ��2�֣���8��)
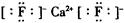 (ÿ��2�֣���8��)
(ÿ��2�֣���8��)����Ԫ�صĽṹ�����ʿ�֪��A��B��C��D��E�ֱ���F��Na��Al��Cl��Ca��
��1����������Ӵ�����������Էе�����Ȼ���ġ���������Ų���ͬ�����������뾶��ԭ���������������С����˷����Ӱ뾶���������Ӱ뾶��
��2������ԭ��������13�����������ڱ��е�λ���ǵ������ڡ���A�塣
��3�����ݾ����ṹ��֪�����еĸ�������8��1/8+6��1/2��4����������8�������Ի�ѧʽΪCaF2���������Ӽ������Ե���ʽΪ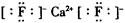 ��
��
��1����������Ӵ�����������Էе�����Ȼ���ġ���������Ų���ͬ�����������뾶��ԭ���������������С����˷����Ӱ뾶���������Ӱ뾶��
��2������ԭ��������13�����������ڱ��е�λ���ǵ������ڡ���A�塣
��3�����ݾ����ṹ��֪�����еĸ�������8��1/8+6��1/2��4����������8�������Ի�ѧʽΪCaF2���������Ӽ������Ե���ʽΪ
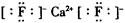 ��
��
��ϰ��ϵ�д�
 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
�����Ŀ



 ���е�
���е� ����
���� ����������������ˮ��Ҳ���������ѡ���ͪ���л��ܼ����ݴ��ж�GE3�ľ�������Ϊ________________��
����������������ˮ��Ҳ���������ѡ���ͪ���л��ܼ����ݴ��ж�GE3�ľ�������Ϊ________________�� ��ʾ����λ�ã��ڴ���������⡢������ȴ�������ӦSi����
��ʾ����λ�ã��ڴ���������⡢������ȴ�������ӦSi���� ��ʾ����λ�á�
��ʾ����λ�á�


