��Ŀ����
��Ԫ��ȱ���������״�ټ�����ΪԤ����ȱ���ҹ��������涨ÿǧ��ʳ����Ӧ����40��50mg KIO3��ij��ѧ�С�����ʵ�飬��֤ijʳ����Ʒ���Ƿ�ͼ���ʳ���Ƿ�Ϊ�ϸ��Ʒ��
��1���������ʵ�鱨�棺
��2���ӵ�ʳ�εİ�װ���ϱ���ʳ�÷�������ʳƷ�����룮��ԭ���ǣ�
��3����ͬѧȡ��ʳ����Ʒ100.0g��ȫ�ܽ���ˮ�У�Ȼ������������ữ�ĵ���-KI��Һ�������Һ����ɫ����Ӧ�����ӷ���ʽΪ
Ϊ��һ��ȷ֤��Ʒ�Ƿ�Ϊ�ϸ��Ʒ����ͬѧ����0.0100mol?L-1��Na2S2O3��Һ�ζ�������ȥ10.00mLʱ��ɫ�պ���ȥ����Ӧ����ʽΪI2+2S2O32-�T2I-+S4O62-����ͨ������üӵ�ʳ����KIO3�ĺ���Ϊ
��4��KIO3���õ��ķ����Ƶã�ԭ���ǣ���ʯīΪ�������Բ����Ϊ��������һ���¶Ⱥ͵����µ��KI��Һ���ܷ�Ӧ����ʽΪKI+3H2O�TKIO3+3H2�����������ĵ缫��ӦʽΪ
��1���������ʵ�鱨�棺
| ʵ�鲽�� | ʵ������ | ʵ����ۣ�����2�����ӷ���ʽ��ʾ�� |
| 1��ȡʳ��������һ֧�Թ��У���������������ˮ���� | ������ȫ�ܽ� | ʳ�μ�����ض�������ˮ |
| 2����������Һ�еμ�������������������Һ���� | ��Һ�����ػ�ɫ | 2IO3-+5HSO3-�TI2+5SO42-+3H++H2O 2IO3-+5HSO3-�TI2+5SO42-+3H++H2O |
| 3�� ����������Һ�еμӵ�����Һ���� ����������Һ�еμӵ�����Һ���� |
��Һ�����ɫ | ����������� ����������� |
��ֹKIO3�ֽ�
��ֹKIO3�ֽ�
����3����ͬѧȡ��ʳ����Ʒ100.0g��ȫ�ܽ���ˮ�У�Ȼ������������ữ�ĵ���-KI��Һ�������Һ����ɫ����Ӧ�����ӷ���ʽΪ
5I-+IO3-+6H+�T3I2+3H2O
5I-+IO3-+6H+�T3I2+3H2O
��Ϊ��һ��ȷ֤��Ʒ�Ƿ�Ϊ�ϸ��Ʒ����ͬѧ����0.0100mol?L-1��Na2S2O3��Һ�ζ�������ȥ10.00mLʱ��ɫ�պ���ȥ����Ӧ����ʽΪI2+2S2O32-�T2I-+S4O62-����ͨ������üӵ�ʳ����KIO3�ĺ���Ϊ
35.7
35.7
mg/kg������һλС�������ɴ˿��ж���ʳ��Ϊ���ϸ�
���ϸ�
����ϸ��ϸ���Ʒ���ζ�ʱʢ��Na2S2O3��Һ����ʽ
��ʽ
�ζ��ܣ����ʽ����ʽ������4��KIO3���õ��ķ����Ƶã�ԭ���ǣ���ʯīΪ�������Բ����Ϊ��������һ���¶Ⱥ͵����µ��KI��Һ���ܷ�Ӧ����ʽΪKI+3H2O�TKIO3+3H2�����������ĵ缫��ӦʽΪ
I--6e-+3H2O�TIO3-+6H+
I--6e-+3H2O�TIO3-+6H+
����������pHֵ����
����
���������С�����䡱����������1��ʳ�μ����������ˮ�����Һ�У��μ�������������������Һ��������ܹ��������������ӷ�Ӧ���ɵ��ʵ����������ӣ���Һ�����ɫ�����ݵ�������ж�ʵ���м����˵�����Һ���μӵ�����Һ�����������������
��2�����ݵ�����ȶ��Բ���з�����
��3��IO3-���������ԣ�����������������л�ԭ�Ե�I-����������ԭ��Ӧ����I2��
��4�����ݷ�Ӧ����ʽ�ҳ���ϵʽIO3-��3I2��6S2O32-�������õζ����ݼ��������ص��������������1Kgʳ�����к��еĵ�������������ݼ������ж������Ƿ�ϸ��������������ǿ�������Σ���Һ��ʾ���ԣ�
��5����ⷨ��KIO3�У������ĵ缫��ӦʽΪI-+3H2O-6e-=IO3-+6H+��������ӦʽΪ��2H2O+2e-=H2��+2OH-���Դ��жϣ�
��2�����ݵ�����ȶ��Բ���з�����
��3��IO3-���������ԣ�����������������л�ԭ�Ե�I-����������ԭ��Ӧ����I2��
��4�����ݷ�Ӧ����ʽ�ҳ���ϵʽIO3-��3I2��6S2O32-�������õζ����ݼ��������ص��������������1Kgʳ�����к��еĵ�������������ݼ������ж������Ƿ�ϸ��������������ǿ�������Σ���Һ��ʾ���ԣ�
��5����ⷨ��KIO3�У������ĵ缫��ӦʽΪI-+3H2O-6e-=IO3-+6H+��������ӦʽΪ��2H2O+2e-=H2��+2OH-���Դ��жϣ�
����⣺��1������ʳ�μ����������ˮ�����Һ�У��μ�������������������Һ��������ܹ��������������ӷ�Ӧ���ɵ��ʵ����������ӣ���Һ�����ɫ�����ݵ�������ж�ʵ���м����˵�����Һ���μӵ�����Һ�����������������
�ʴ�Ϊ��
��2��������������ȶ��Բ�������ֽ⣬����ʳ��ʱ��ʳƷ�����룬
�ʴ�Ϊ����ֹKIO3�ֽ⣻
��3��IO3-���������ԣ�����������������л�ԭ�Ե�I-����������ԭ��Ӧ����I2����Ӧ�����ӷ���ʽΪIO3-+5I-+6H+=3I2+3H2O��
�ʴ�Ϊ��IO3-+5I-+6H+=3I2+3H2O��
��4�����ݷ�ӦIO3-+5I-+6H+=3I2+3H2O���Լ�I2+2S2O32-����ɫ����2I-+S4O62-����ɫ�����ó���Ӧ�Ĺ�ϵʽIO3-��3I2��6S2O32-��
��n��Na2S2O3��=0.010mol/L��0.012L=0.00012mol��
��IO3-��3I2��6S2O32-
1 6
n��IO3-�� 0.00010mol
��ã�n��IO3-��=
��0.00010mol��
m��KIO3��=
��0.00010mol��214g/mol=0.00428g=3.57mg��
����1kgʳ���У�m��KIO3��=10��4.28mg=35.7mg��40mg��
���Ը�ʳ�β��ϸ�
�����������������ǿ�������Σ�����Һ��ʾ���ԣ�����Ӧ��ʹ�ü�ʽ�ζ���ʢװ��
�ʴ�Ϊ��5I-+IO3-+6H+�T3I2+3H2O����35.7�����ϸ� ��ʽ��
��5����ⷨ��KIO3�У���������������Ӧ���缫��ӦʽΪI-+3H2O-6e-=IO3-+6H+��������ӦʽΪ��2H2O+2e-=H2��+2OH-������PH����
�ʴ�Ϊ��I--6e-+3H2O�TIO3-+6H+��������
�ʴ�Ϊ��
| ʵ�鲽�� | ʵ������ | ʵ����ۣ�����2�� ���ӷ���ʽ��ʾ�� |
| 2IO3-+5HSO3-�TI2+5SO42-+3H++H2O | ||
| ����������Һ�еμӵ�����Һ���� | ����������� |
�ʴ�Ϊ����ֹKIO3�ֽ⣻
��3��IO3-���������ԣ�����������������л�ԭ�Ե�I-����������ԭ��Ӧ����I2����Ӧ�����ӷ���ʽΪIO3-+5I-+6H+=3I2+3H2O��
�ʴ�Ϊ��IO3-+5I-+6H+=3I2+3H2O��
��4�����ݷ�ӦIO3-+5I-+6H+=3I2+3H2O���Լ�I2+2S2O32-����ɫ����2I-+S4O62-����ɫ�����ó���Ӧ�Ĺ�ϵʽIO3-��3I2��6S2O32-��
��n��Na2S2O3��=0.010mol/L��0.012L=0.00012mol��
��IO3-��3I2��6S2O32-
1 6
n��IO3-�� 0.00010mol
��ã�n��IO3-��=
| 1 |
| 6 |
m��KIO3��=
| 1 |
| 6 |
����1kgʳ���У�m��KIO3��=10��4.28mg=35.7mg��40mg��
���Ը�ʳ�β��ϸ�
�����������������ǿ�������Σ�����Һ��ʾ���ԣ�����Ӧ��ʹ�ü�ʽ�ζ���ʢװ��
�ʴ�Ϊ��5I-+IO3-+6H+�T3I2+3H2O����35.7�����ϸ� ��ʽ��
��5����ⷨ��KIO3�У���������������Ӧ���缫��ӦʽΪI-+3H2O-6e-=IO3-+6H+��������ӦʽΪ��2H2O+2e-=H2��+2OH-������PH����
�ʴ�Ϊ��I--6e-+3H2O�TIO3-+6H+��������
���������⿼���Ϊ�ۺϣ��漰�绯ѧ�����ӷ���ʽ����д�Լ����ʺ����IJⶨ����Ŀ�Ѷ��еȣ����ʱע����շ�Ӧ�Ĺ�ϵʽ��

��ϰ��ϵ�д�
�����Ŀ
��Ԫ��ȱ���������״�ټ�����ΪԤ����ȱ���ҹ��������涨ÿǧ��ʳ����Ӧ����40��50mg KIO3��ij��ѧ�С�����ʵ�飬��֤ijʳ����Ʒ���Ƿ�ͼ���ʳ���Ƿ�Ϊ�ϸ��Ʒ��
��1���������ʵ�鱨�棺
| ʵ�鲽�� | ʵ������ | ʵ����ۣ�����2�����ӷ���ʽ��ʾ�� |
| 1��ȡʳ��������һ֧�Թ��У���������������ˮ���� | ������ȫ�ܽ� | ʳ�μ�����ض�������ˮ |
| 2����������Һ�еμ�������������������Һ���� | ��Һ�����ػ�ɫ | ______ |
| 3��______ | ��Һ�����ɫ | ______ |
��3����ͬѧȡ��ʳ����Ʒ100.0g��ȫ�ܽ���ˮ�У�Ȼ������������ữ�ĵ���-KI��Һ�������Һ����ɫ����Ӧ�����ӷ���ʽΪ______��
Ϊ��һ��ȷ֤��Ʒ�Ƿ�Ϊ�ϸ��Ʒ����ͬѧ����0.0100mol?L-1��Na2S2O3��Һ�ζ�������ȥ10.00mLʱ��ɫ�պ���ȥ����Ӧ����ʽΪI2+2S2O32-�T2I-+S4O62-����ͨ������üӵ�ʳ����KIO3�ĺ���Ϊ______mg/kg������һλС�������ɴ˿��ж���ʳ��Ϊ______����ϸ��ϸ���Ʒ���ζ�ʱʢ��Na2S2O3��Һ��______�ζ��ܣ����ʽ����ʽ����
��4��KIO3���õ��ķ����Ƶã�ԭ���ǣ���ʯīΪ�������Բ����Ϊ��������һ���¶Ⱥ͵����µ��KI��Һ���ܷ�Ӧ����ʽΪKI+3H2O�TKIO3+3H2�����������ĵ缫��ӦʽΪ______����������pHֵ______���������С�����䡱��

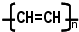 ��������
��������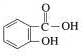 ������Ϊˮ���ᣬ��ҽѧ�Ͼ���
������Ϊˮ���ᣬ��ҽѧ�Ͼ���