��Ŀ����
����Ŀ����1������25�����ۼ�����������ʽ________________���˷��Ӿ���ͬ���칹����������һ����Ľṹ��������������ԭ����ȫ��ͬ��д����������_______________________��
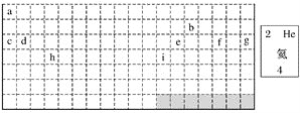
��2���л���ṹ���ü���ʽ�������ʽ��ת�۴���ĩ��Ϊ̼ԭ��(Hԭ��ʡ��)���߾��Ǽ�������ԭ�ӱ���ע������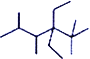 ��ϵͳ�������Ը��л����������___________________________�����ǵ�ϩ���������ӳɺ�IJ����ϩ�����ܵĽṹ��________________�֡�
��ϵͳ�������Ը��л����������___________________________�����ǵ�ϩ���������ӳɺ�IJ����ϩ�����ܵĽṹ��________________�֡�
��3������ʽΪC6H12��ijϩ��������̼ԭ�Ӷ���ͬһ��ƽ���ϣ���ṹ��ʽΪ___________________��
��4���ױ������ϵ�һ����ԭ�ӱ���3��̼ԭ�ӵ�������ȡ�������ò�����__________________��
���𰸡� C8H18 2,2,3,3���ļ����� 2,2,4,5-�ļ�-3,3-���һ����� 4 (CH3)2C=C(CH3)2 6
����������1����������CnH2n��2�й��ۼ��ĸ�������(2n��2)��̼�ⵥ�������а�n��̼������״��(n��1)��̼̼��������(n��1)��(2n��2)��25������n��8��������D�ķ���ʽΪC8H18���˷�����������ԭ����ȫ��ͬ�Ľṹ��ʽΪ��(CH3)3CC(CH3)3 ������Ϊ��2,2,3,3-�ļ���������Ϊ��C8H18 ��2,2,3,3���ļ�����
��2�����ļ���ʽ�н��㡢�˵�Ϊ̼ԭ�ӣ���Hԭ�ӱ���C���ļ۽ṹ�����л������ʽ��֪���л���Ľṹ��ʽ������ϵͳ�������Ը��л�������Ϊ��2,2,4,5-�ļ�-3,3-���һ����飻���л����������ɵ�ϩ���������ӳɺ�IJ����ԭ�ɵ�ϩ��ʱ���ڵ�����̼ԭ�Ӹ�ȥ��һ����ԭ�ӣ���4�ֵ�ϩ������Ϊ��2,2,4,5-�ļ�-3,3-���һ����飻4
��3��������ϩ��ƽ���ͽṹ������ϩ�����е���ԭ�ӻ��ɼ�ʱ����������2��3-����-2-��ϩ�������������̼ԭ�Ӷ���ͬһƽ���ϣ���ṹ��ʽΪ��(CH3)2C=C(CH3)2 �� ��Ϊ��(CH3)2C=C(CH3)2
��4���ױ���������5����ԭ�ӿɱ�ȡ������ֻ���ڡ��䡢���������������3��̼ԭ�ӵ������2��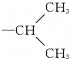 ��-CH2CH2CH3����Ӧ������һԪȡ�����Ϊ��6
��-CH2CH2CH3����Ӧ������һԪȡ�����Ϊ��6

����Ŀ����֪A��g��+B��g��![]() C��g��+D��g����Ӧ��ƽ�ⳣ�����¶ȵĹ�ϵ���£�
C��g��+D��g����Ӧ��ƽ�ⳣ�����¶ȵĹ�ϵ���£�
�¶�/�� | 700 | 800 | 830 | 1000 | 1200 |
ƽ�ⳣ�� | 1.7 | 1.1 | 1.0 | 0.6 | 0.4 |
830��ʱ����һ��2L���ܱ������г���0.2mol��A��0.8mol��B����Ӧ��ʼ4s��A��ƽ����Ӧ����v��A��=0.005 mol/��L��s��������˵����ȷ����
A. 4sʱc��B��Ϊ0.78 mol/L
B. �÷�ӦAH>0
C. 830���ƽ��ʱ��A��ת����Ϊ20%
D. 1200��ʱ��ӦC��g��+D��g��![]() A��g��+B��g����ƽ�ⳣ��Ϊ2.5
A��g��+B��g����ƽ�ⳣ��Ϊ2.5