��Ŀ����
�����8�֣��±���Ԫ�����ڱ���һ���֣���ش��й����⣺
��1��hԪ����kԪ���γɵĻ�����ĵ���ʽ��_____________________��
��2��nԪ����fԪ�����ߺ˵����֮����_____________________��
��3�����������У�ԭ�Ӱ뾶�����ǣ�ϡ��������⣩________________��
��4���Ʋ�Si��N����⻯����ȶ���ǿ����ϵ____________���ѧʽ����
��5��c2a4��n�ĵ��ʷ�Ӧ�Ļ�ѧ����ʽ______________________________��
��6��д��c5a12������ͬ���칹��Ľṹ��ʽ_________________________��
| | IA | ��A | | ��A | ��A | VA | ��A | ��A | 0 | |
| 1 | a | | b | |||||||
| 2[ | | | | | c | d | e | f | g | |
| 3 | h | | i | j | | | k | l | ||
| 4 | m | | | | | ] | n | | ||
��2��nԪ����fԪ�����ߺ˵����֮����_____________________��
��3�����������У�ԭ�Ӱ뾶�����ǣ�ϡ��������⣩________________��
��4���Ʋ�Si��N����⻯����ȶ���ǿ����ϵ____________���ѧʽ����
��5��c2a4��n�ĵ��ʷ�Ӧ�Ļ�ѧ����ʽ______________________________��
��6��д��c5a12������ͬ���칹��Ľṹ��ʽ_________________________��
��1�� ��2��26 ��3��Na ��4��NH3 > SiH4 ��5��CH2=CH2+Br2 ��CH2BrCH2Br
��2��26 ��3��Na ��4��NH3 > SiH4 ��5��CH2=CH2+Br2 ��CH2BrCH2Br
��6��CH3CH2CH2CH2CH3 CH3CH2CH(CH3)2 C(CH3)4
 ��2��26 ��3��Na ��4��NH3 > SiH4 ��5��CH2=CH2+Br2 ��CH2BrCH2Br
��2��26 ��3��Na ��4��NH3 > SiH4 ��5��CH2=CH2+Br2 ��CH2BrCH2Br��6��CH3CH2CH2CH2CH3 CH3CH2CH(CH3)2 C(CH3)4
�������֪aΪH��bΪHe��cΪC��dΪN��eΪO��fΪF��gΪNe��hΪNa��iΪAl��jΪSi��kΪCl��lΪAr��mΪK��nΪBr��hԪ����kԪ���γɵĻ�����Ϊ�Ȼ��ƣ�nԪ����fԪ�����ߺ˵����֮����26. �ȶ���NH3 > SiH4��

��ϰ��ϵ�д�
�����Ŀ
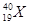 ��
��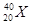
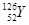 ��
��