��Ŀ����
ȡm1gij������ĩ����M��Z����Ԫ����ɣ���������ʵ�顣���÷�ĩ������̼�۳�ֻ�ϣ�ƽ���ڷ�Ӧ��a�У�bƿ��ʢ��������ʯ��ˮ����ͼ�������������������ԡ�

ʵ�鿪ʼʱ���Ƚ��в���G����һ��ʱ����ȷ�Ӧ��a���۲쵽���ڷ������ҷ�Ӧ���������������ɡ�ͬʱ��bƿ����Һ�г��ְ�ɫ���ǡ���һϵ�вⶨ��ȷ����Ӧ��ȫ��ֹͣ���ȣ�����ͨ������ֱ����Ӧ����ȴ����ʱ�����е����������̳�����ɫ����M���ٽ��з��롢�ᴿ���������õ�m2g������M����m1=1.38m2���������������ش����⣺
(1)�÷�ĩ�Ļ�ѧʽ��_________ ____������G��______________________��
____������G��______________________��
(2)ֹͣ����ǰ�Ƿ���Ҫ�ȹر�ֹˮ��K��_________��ԭ��_____________��
(3)��Ӧ��a�в����������ʵĻ�ѧ����ʽΪ��__________________________��
 (4)��ʵ���β���Ƿ��账����_______�����账������ش���δ������粻�账����
(4)��ʵ���β���Ƿ��账����_______�����账������ش���δ������粻�账����
��˵�����ɡ�_______________________________________________��
(5)����Ŀ��һϵ�вⶨ���У�������_____________ʱ�����ȷ����Ӧ���з�Ӧ����ȫ��
(6)ijͬѧ����ʵ��ʱ��bƿ���յõ��˳�����Һ���������Һ�м�����ij���ʣ���Һ�ֳ����˻��ǡ�����д���ֻ��ǵ����ӷ���ʽ��___________________________��

ʵ�鿪ʼʱ���Ƚ��в���G����һ��ʱ����ȷ�Ӧ��a���۲쵽���ڷ������ҷ�Ӧ���������������ɡ�ͬʱ��bƿ����Һ�г��ְ�ɫ���ǡ���һϵ�вⶨ��ȷ����Ӧ��ȫ��ֹͣ���ȣ�����ͨ������ֱ����Ӧ����ȴ����ʱ�����е����������̳�����ɫ����M���ٽ��з��롢�ᴿ���������õ�m2g������M����m1=1.38m2���������������ش����⣺
(1)�÷�ĩ�Ļ�ѧʽ��_________
 ____������G��______________________��
____������G��______________________��(2)ֹͣ����ǰ�Ƿ���Ҫ�ȹر�ֹˮ��K��_________��ԭ��_____________��
(3)��Ӧ��a�в����������ʵĻ�ѧ����ʽΪ��__________________________��
 (4)��ʵ���β���Ƿ��账����_______�����账������ش���δ������粻�账����
(4)��ʵ���β���Ƿ��账����_______�����账������ش���δ������粻�账������˵�����ɡ�_______________________________________________��
(5)����Ŀ��һϵ�вⶨ���У�������_____________ʱ�����ȷ����Ӧ���з�Ӧ����ȫ��
(6)ijͬѧ����ʵ��ʱ��bƿ���յõ��˳�����Һ���������Һ�м�����ij���ʣ���Һ�ֳ����˻��ǡ�����д���ֻ��ǵ����ӷ���ʽ��___________________________��
��11�֣�
(1)Fe3O4������ͨ�뵪����2�֣�
(2)����Ҫ����Ϊֹͣ���Ⱥ��Լ���ͨN2�����ᷢ����������2�֣�
(3) Fe3O4��4C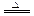 3Fe��4CO������F
3Fe��4CO������F e3O4��2C
e3O4��2C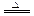 3Fe��2CO2����
3Fe��2CO2����
��Fe3O4��4CO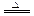 3Fe��4CO2�� ��1�֣�
3Fe��4CO2�� ��1�֣�
(4)��Ҫ��������Ϊ�������ж���CO�� ��1�֣�
������һ�����ȵ�װ��CuO��ĩ�ķ�Ӧ��(���һ����ȼ�ľƾ��ƣ�����һ�����ռ����塣����������ͬ������)����1 �֣�
�֣�
(5)���γ�����Ӧ�ܼ���Ӧ������������IJ�ֵ������0.001g����2�֣�
(6)Ca2++HCO3��+OH����CaCO3��+H2O��2�֣�������������Ҳ���֣����Ba(OH)2
(1)Fe3O4������ͨ�뵪����2�֣�
(2)����Ҫ����Ϊֹͣ���Ⱥ��Լ���ͨN2�����ᷢ����������2�֣�
(3) Fe3O4��4C
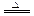 3Fe��4CO������F
3Fe��4CO������F e3O4��2C
e3O4��2C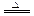 3Fe��2CO2����
3Fe��2CO2������Fe3O4��4CO
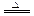 3Fe��4CO2�� ��1�֣�
3Fe��4CO2�� ��1�֣�(4)��Ҫ��������Ϊ�������ж���CO�� ��1�֣�
������һ�����ȵ�װ��CuO��ĩ�ķ�Ӧ��(���һ����ȼ�ľƾ��ƣ�����һ�����ռ����塣����������ͬ������)����1
 �֣�
�֣�(5)���γ�����Ӧ�ܼ���Ӧ������������IJ�ֵ������0.001g����2�֣�
(6)Ca2++HCO3��+OH����CaCO3��+H2O��2�֣�������������Ҳ���֣����Ba(OH)2
��

��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ

 ��
�� 
 ��3���ӻ��������ĽǶȿ��ǣ���
��3���ӻ��������ĽǶȿ��ǣ��� ��Ϊ��ʵ�����Ƹ���θĽ�?��д��һ�ָĽ�����:
��Ϊ��ʵ�����Ƹ���θĽ�?��д��һ�ָĽ�����: ��1������һ�����ʵ���Ũ�ȵ�BaC12��Һ�����Ѿ������õ� gBaC12��������0.4mol��L��lBaCl2��Һ250 mL������Ҫ������Ϊ��Ͳ�� ��
��1������һ�����ʵ���Ũ�ȵ�BaC12��Һ�����Ѿ������õ� gBaC12��������0.4mol��L��lBaCl2��Һ250 mL������Ҫ������Ϊ��Ͳ�� ��
 ʵ���д���ƿ�в��ٲ�����������ӵ���A�˻�������һ�����Ŀ�������������Ŀ���� ��
ʵ���д���ƿ�в��ٲ�����������ӵ���A�˻�������һ�����Ŀ�������������Ŀ���� ��

 aCl��Һ(NaHCO3)���Լ� �����ӷ���ʽ ��
aCl��Һ(NaHCO3)���Լ� �����ӷ���ʽ ��