��Ŀ����
 A��B��C��N��O��Si��Fe��Ԫ�ؼ��仯��������Ҫ��Ӧ�ã�
A��B��C��N��O��Si��Fe��Ԫ�ؼ��仯��������Ҫ��Ӧ�ã���1����������Ԫ�ص�ԭ���У�δ�ɶԵ���������Ԫ����
��2����Fe��ͬһ���ڵĸ���Ԫ���У�����������Ϊ1��Ԫ����
��3��CԪ�����γ��л������ҪԪ�أ����з����к���sp1��sp3�ӻ���ʽ����
a��
 b��CH4 c��CH2=CHCH3 d��CH3C��CH
b��CH4 c��CH2=CHCH3 d��CH3C��CH��4��C��Si��Nԭ�ӵ縺���ɴ�С��˳����
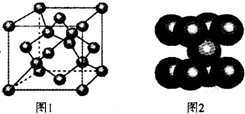
��5��һ�������£�CԪ�ؿ��γɶ��־��壮ͼ1������ij�־����һ���������þ����к���
��6��ͼ2Ϊ������ij�־���ľ����ṹ����֪�þ�����ܶ�Ϊa gΪ�����ӵ�������ֵ����þ��������Ϊ
���㣺�����ļ���,λ�ýṹ���ʵ����ϵӦ��
ר�⣺Ԫ����������Ԫ�����ڱ�ר��,��ѧ���뾧��ṹ
��������1���������������Ų�ʽ��δ�ɶԵ������жϣ�
��2��������Fe��ͬһ���ڵĸ���Ԫ���У���������Ų�ʽ�жϣ�
��3�����ݼ۲���ӶԻ�������ȷ�����ӻ���ʽ��
��4������Ԫ�صĵ縺�Եݱ�����жϣ����ݵ�һ�����ܵݱ�����жϣ�
��5�����þ�̯������̼ԭ�Ӹ�����
��6�������ܶȺ;����������㣮
��2��������Fe��ͬһ���ڵĸ���Ԫ���У���������Ų�ʽ�жϣ�
��3�����ݼ۲���ӶԻ�������ȷ�����ӻ���ʽ��
��4������Ԫ�صĵ縺�Եݱ�����жϣ����ݵ�һ�����ܵݱ�����жϣ�
��5�����þ�̯������̼ԭ�Ӹ�����
��6�������ܶȺ;����������㣮
���
�⣺��1��Cԭ����δ�ɶԵ�������2��Nԭ����δ�ɶԵ�������3��Siԭ����δ�ɶԵ�������2��Feԭ����δ�ɶԵ�������4���ʴ�Ϊ��Fe��
��2��Feλ�ڵ������ڣ�����Ԫ���У�Cr�۵����Ų�ʽΪ��3d54s1��Cu�۵����Ų�ʽΪ��3d104s1��������������Ϊ1��Ԫ����Cu��Cr���ʴ�Ϊ��Cu��Cr��
��3��a����̼ԭ����4�����۵���������̼ԭ�Ӳ���sp3�ӻ���������̼ԭ�Ӳ���sp2�ӻ����ʲ����ϣ�
b��������̼ԭ�Ӳ���sp3�ӻ����ʲ����ϣ�
c��CH2=CHCH3�ļ�̼ԭ�Ӳ���sp3�ӻ�������̼̼˫����̼ԭ�Ӳ���sp2�ӻ����ʲ����ϣ�
d��CH3CH2C��CH�ļ����Ǽ�̼ԭ�Ӳ���sp3�ӻ�������̼̼������̼ԭ�Ӳ���sp�ӻ����ʷ��ϣ�
��ѡd��
��4��ͬһ����Ԫ��֪��Ԫ�صĵ縺������ԭ���������������С��ͬһ����Ԫ���У�Ԫ�صĵ縺������ԭ���������������������C��Si��Nԭ�ӵ縺���ɴ�С��˳����N��C��Si��ͬһ����Ԫ���У�Ԫ�صĵ�һ����������ԭ����������������������ƣ�����NԪ�أ�2p������������Ϊ�ȶ�����һ�����ܴ���O����һ�����ܴ�С����˳��Ϊ��B��C��O��N
��5������ͼ1������֪̼ԭ��λ�ڶ��㡢���ĺ����ڣ���ĿΪ8��
+6��
+4=8�������к���8��̼ԭ�ӣ�
�ʴ�Ϊ��8��
��6����������Ϊ���������������к�����ԭ����ĿΪ8��
+1=2��������Ϊ
�������Ϊ
=
��
�ʴ�Ϊ��
��
��2��Feλ�ڵ������ڣ�����Ԫ���У�Cr�۵����Ų�ʽΪ��3d54s1��Cu�۵����Ų�ʽΪ��3d104s1��������������Ϊ1��Ԫ����Cu��Cr���ʴ�Ϊ��Cu��Cr��
��3��a����̼ԭ����4�����۵���������̼ԭ�Ӳ���sp3�ӻ���������̼ԭ�Ӳ���sp2�ӻ����ʲ����ϣ�
b��������̼ԭ�Ӳ���sp3�ӻ����ʲ����ϣ�
c��CH2=CHCH3�ļ�̼ԭ�Ӳ���sp3�ӻ�������̼̼˫����̼ԭ�Ӳ���sp2�ӻ����ʲ����ϣ�
d��CH3CH2C��CH�ļ����Ǽ�̼ԭ�Ӳ���sp3�ӻ�������̼̼������̼ԭ�Ӳ���sp�ӻ����ʷ��ϣ�
��ѡd��
��4��ͬһ����Ԫ��֪��Ԫ�صĵ縺������ԭ���������������С��ͬһ����Ԫ���У�Ԫ�صĵ縺������ԭ���������������������C��Si��Nԭ�ӵ縺���ɴ�С��˳����N��C��Si��ͬһ����Ԫ���У�Ԫ�صĵ�һ����������ԭ����������������������ƣ�����NԪ�أ�2p������������Ϊ�ȶ�����һ�����ܴ���O����һ�����ܴ�С����˳��Ϊ��B��C��O��N
��5������ͼ1������֪̼ԭ��λ�ڶ��㡢���ĺ����ڣ���ĿΪ8��
| 1 |
| 8 |
| 1 |
| 2 |
�ʴ�Ϊ��8��
��6����������Ϊ���������������к�����ԭ����ĿΪ8��
| 1 |
| 8 |
| 2��56 |
| g |
| ||
| a |
| 112 |
| ag |
�ʴ�Ϊ��
| 112 |
| ag |
���������⿼�龧���ļ��㡢������ԭ�Ӹ�����ȷ�����縺�Դ�С���жϵ�֪ʶ�㣬���þ�̯����Ԫ����������������ɣ��Ѷ��еȣ�

��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ
����˵����ȷ���ǣ�������
| A��ʵ���ҴӺ�����ȡ���ʵ�ķ��������ǣ�ȡ�������ա��ܽ⡢���ˡ���ȡ |
| B�������Ӵ���ʱ�������������Ĥ�ױ��ƻ�����˺�������Ʒ����ֱ�ӷ������������� |
| C�����Ҵ���ŨH2SO4�Ʊ���ϩʱ������ˮԡ���ȿ��Ʒ�Ӧ���¶� |
| D����֬�ڼ��������µ�ˮ�ⷴӦ��Ϊ��֬���⻯���ֳ�Ϊ��֬��Ӳ�� |
�ں��¡����ݵ��ܱ������н��з�ӦA��g��?B��g��+C��g��������Ӧ���Ũ����2mol/L����0.8mol/L��20s����ô��Ӧ��Ũ����0.8mol/L����0.2mol/L���跴Ӧʱ��Ϊ��������
| A��10s | B������10s |
| C��С��10s | D�����ж� |
���з�Ӧ�У����ڼӳɷ�Ӧ���ǣ�������
| A��������������Ӧ��ȡһ�ȼ��� |
| B���������Ҵ���Ӧ��ȡ�������� |
| C�������뱥����ˮ��Ӧ�������屽�� |
| D����ϩ���Ȼ��ⷴӦ��ȡ������ |
�������ʷе�ıȽϣ���ȷ���ǣ�������
| A�����飾���� |
| B�������飾������ |
| C�����飾�Ҵ� |
| D���Ҵ����Ҷ��� |
 �����Ǽ��ֲ�ͬ���͵�©������;�в��죮
�����Ǽ��ֲ�ͬ���͵�©������;�в��죮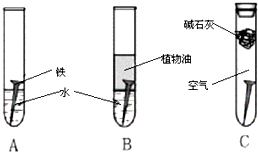 ij�о�С�������������о���
ij�о�С�������������о���