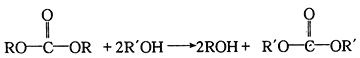��Ŀ����
����Ŀ����Ԫ�صĵ��ʼ��仯�����ڿ�ѧ�о�����ũҵ������ũҩ���Ʊ���ʹ�õȷ�����й㷺��;����
��������Ӧ�ûش��й�������
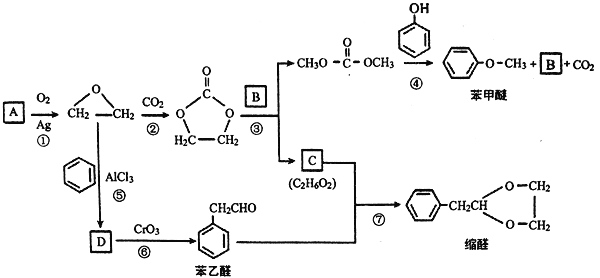
(1)��֪��������S2��S4��S6��S8��Sn�ȶ�����ʽ����Sn������Sԭ����S��S�����γ������������n
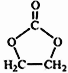
Ԫ�����Ի���S8�İ�Ԫ���ṹʽ___________��
(2)��ɫũҩ��ʯ��ϼ�������Ч�ɷ�Ϊ����(CaS5)����������(CaS2O3),���ɵ��������ʯ���ڼ����������Ƶã��÷�Ӧ�Ļ�ѧ����ʽΪ(��Ӧ��������Ҫ����S4��ʾ)��____________��
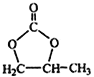
��֪����Ϊ���ӻ��������Sԭ����S��S�������ӳɶ��������γ���2�۵�ԭ���ţ��Ի������Ƶĵ���ʽ_________________��
(3)��ѧ��̽������Ǵ����к���һ�ֳ�Ϊ���ʵ���ɣ��仯ѧʽΪCOS���ṹ��������̼�������������ʿ���Ϊһ��Ѭ�������ܷ�ֹijЩ���桢�߳��Σ�������������±�����ع��ۼ��ļ������ݣ�
���ۼ� | C=O | C=S | H��O | H��S |
����/kJ.mol-1 | 745 | 536 | 464 | 339 |
�����йط�Ӧԭ��д������������ˮ������Ӧ����CO2��H2S���Ȼ�ѧ����ʽ��______________.
(4)S4��������ɱ�����Ϳ�������У�����H2S2��ȼ�ϵ�ػ��,��װ������ͼ��ʾ��

��H2S2��������________��
���缫bΪ________ (ѡ������������ ��������)��
���缫a�Ϸ����ĵ缫��ӦΪ��_______________��
(5)һ��������(S2O)��������һ����ɫ�����ȶ������壬ʵ���ҿ���S8������ͭ�����Ƶã�ͬʱ������ͭ��SO2��ע���������������������ʵ����������뽵�۵���Ҳ�ǵ�������������
���Ʊ���Ӧ�Ļ�ѧ����ʽΪ________��
��֪S2O����ʱ�ֽ��������ֺ���ij���������������S2O����Ԫ���ļ�̬������д���÷ֽⷴӦ�Ļ�ѧ����ʽ_____________________��
���𰸡� ![]() ��
�� ![]() 3Ca(OH)2 +3S4
3Ca(OH)2 +3S4![]() 2CaS5 +CaS2O3 +3H2O
2CaS5 +CaS2O3 +3H2O ![]() COS(g)+H2O(g)=CO2(g)+H2S(g)����H��+41 kJ��mol-1 ������ ���� 4H2S2-8e-��S8 +8H+ 3S8 +12CuO
COS(g)+H2O(g)=CO2(g)+H2S(g)����H��+41 kJ��mol-1 ������ ���� 4H2S2-8e-��S8 +8H+ 3S8 +12CuO![]() 12CuS+4S2O��+4SO2�� 2S2O=SO2+3S
12CuS+4S2O��+4SO2�� 2S2O=SO2+3S
��������(1)��Sn������Sԭ����S��S�����γɡ�����Ρ���nԪ�������S8�İ�Ԫ���ṹʽΪ![]() ��
�� ![]() ��(2)����ԭ���غ�͵��ӵ�ʧ�غ��֪�÷�Ӧ�Ļ�ѧ����ʽΪ3Ca(OH)2 +3S4
��(2)����ԭ���غ�͵��ӵ�ʧ�غ��֪�÷�Ӧ�Ļ�ѧ����ʽΪ3Ca(OH)2 +3S4![]() 2CaS5 +CaS2O3 +3H2O������Ϊ���ӻ��������Sԭ����S��S�������ӳɶ��������γɣ�2�۵�ԭ���ţ������Ƶĵ���ʽΪ
2CaS5 +CaS2O3 +3H2O������Ϊ���ӻ��������Sԭ����S��S�������ӳɶ��������γɣ�2�۵�ԭ���ţ������Ƶĵ���ʽΪ![]() ��(3)��Ӧ�ȵ��ڶϼ����յ��������γɻ�ѧ�����ų������IJ�ֵ����ӦCOS(g)+H2O(g)��CO2(g)+H2S(g)����H��(745+536+464��2-2��745-2��339)kJ/mol=+41 kJ��mol-
��(3)��Ӧ�ȵ��ڶϼ����յ��������γɻ�ѧ�����ų������IJ�ֵ����ӦCOS(g)+H2O(g)��CO2(g)+H2S(g)����H��(745+536+464��2-2��745-2��339)kJ/mol=+41 kJ��mol-
�����йط�Ӧԭ��д������������ˮ������Ӧ����CO2��H2S���Ȼ�ѧ����ʽΪCOS(g)+H2O(g)��CO2(g)+H2S(g)����H��+41 kJ��mol-��(4)�ٸ���H2O2�ǹ���������ж�H2S2�������ǹ����⡣�ڵ缫b����ˮ��˵�������õ����ӣ�Ϊ�������۵缫a��������H2S2����ʧȥ���ӵ�������Ӧ�����������ӹ������ʣ��������ĵ缫��ӦΪ4H2S2-8e-��S8 +8H+��(5)һ�����������������������ʵ������������뽵�۵���Ҳ�ǵ����ʵ��������Ը���ԭ���غ�͵��ӵ�ʧ�غ��֪���Ʊ���Ӧ�Ļ�ѧ����ʽΪ3S8 +12CuO![]() 12CuS+4S2O��+4SO2������֪S2O����ʱ�ֽ��������ֺ���ij������ʣ�SԪ�ػ��ϼ۴�+1�۲��ֽ��͵�0�ۣ��������ߵ�+4�ۣ����Ը÷ֽⷴӦ�Ļ�ѧ����ʽΪ2S2O=SO2+3S��
12CuS+4S2O��+4SO2������֪S2O����ʱ�ֽ��������ֺ���ij������ʣ�SԪ�ػ��ϼ۴�+1�۲��ֽ��͵�0�ۣ��������ߵ�+4�ۣ����Ը÷ֽⷴӦ�Ļ�ѧ����ʽΪ2S2O=SO2+3S��

����Ŀ��1����������һ����ɫҺ�壬�ܶȱ�ˮ�Ĵ�����ˮ���������Ҵ����۵�Ϊ5.5�棬�е�Ϊ267�档1����(�����뱽������)���۵�Ϊ96�棬�е�Ϊ278�棬����ˮ���������Ҵ����Ҵ��ķе�Ϊ78.5�档1�����������������ϣ�Ҳ�ɺϳ��������ϡ�ʵ�����Ʊ�1���������ķ�Ӧԭ�����£�
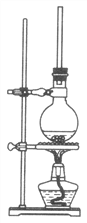

(1)��72g1��������100mL��ˮ�Ҵ��У��ټ���5mLŨ���ᡣ�������Һ������ͼ��ʾ��Բ����ƿ�м��ȳ�ַ�Ӧ��ʵ����ʹ�ù����Ҵ���ԭ����________����ƿ�����ӳ������ܵ���Ҫ������________________��
(2)��Ӧ��������ƿ�е�Һ�嵹����ˮ�У��������õ��л��㡣Ϊ�ᴿ�����������IJ�������������ˮϴ����Һ������10%��NaOH��Һϴ����Һ��������ˮ�Ȼ��Ƹ��ﲢ���ˡ���ȷ��˳����________(�����)��
A���ۢڢܢ١��� B���٢ڢۢ� ����C���ڢ٢ۢ�
(3)ʵ����1���������IJ����뷴Ӧʱ�䡢�¶ȵĹ�ϵ��ͼ��ʾ��ʱ���ӳ����¶����ߣ�1���������IJ����½���ԭ�������__________��____________��
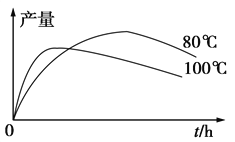
(4)ijͬѧ�Ʋ⾭�ᴿ�IJ�Ʒ���ܻ�����1���ӡ��Ҵ��������ˮ�����ʣ���������·������м��飬����ɱ������ݡ�
ʵ��Ŀ�� | ʵ����� | Ԥ������ͽ��� |
���ý����Ƽ���1���������Ƿ� | ȡ�������ᴿ�IJ�Ʒ���Թ�A�У���������� | ��_________�����Ʒ������ ��__________�����Ʒ���� |
�ڼ��龭�ᴿ�IJ�Ʒ�Ƿ���1���� | _______________ | ��_________������1���ӣ� ��_______________����1���� |